Elements In The Same Group Have Similar
listenit
Apr 01, 2025 · 6 min read
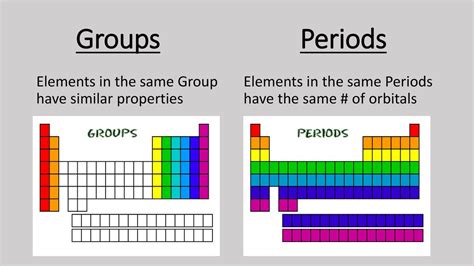
Table of Contents
Elements in the Same Group Have Similar Properties: A Deep Dive into Periodic Trends
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and resulting properties. One of the most fundamental observations is that elements within the same group (vertical column) exhibit striking similarities in their chemical and physical behavior. This isn't a coincidence; it's a direct consequence of their shared electron configuration, specifically the number of valence electrons. Understanding these similarities is crucial for predicting reactivity, bonding patterns, and overall chemical behavior. This article will delve deep into the reasons behind this similarity, exploring various aspects of periodic trends and their implications.
Understanding Electron Configuration and Valence Electrons
The key to understanding why elements in the same group share similar properties lies in their electron configuration. Atoms are comprised of a nucleus containing protons and neutrons, surrounded by electrons arranged in energy levels or shells. The outermost shell contains the valence electrons, which are directly involved in chemical bonding and determine an element's reactivity.
The Significance of Valence Electrons
Valence electrons are the stars of the chemical show. They dictate how an atom will interact with other atoms, forming bonds and creating molecules. Elements in the same group possess the same number of valence electrons. For example, all elements in Group 1 (alkali metals) have one valence electron, all elements in Group 18 (noble gases) have eight valence electrons (except helium, which has two), and so on. This identical number of valence electrons is the primary reason for the observed similarities in properties.
Similar Chemical Properties: Reactivity and Bonding
The similarity in valence electron count translates directly into similar chemical behaviors. This is manifested in several key aspects:
1. Reactivity:
Elements in the same group tend to exhibit similar reactivity patterns. This is because their valence electrons are readily available to participate in chemical reactions. For instance, alkali metals (Group 1) are highly reactive because they easily lose their single valence electron to achieve a stable electron configuration. Halogens (Group 17), on the other hand, are also highly reactive, but they tend to gain an electron to achieve a stable octet. This consistent pattern of reactivity within a group allows chemists to predict how an element might behave based on its group placement.
2. Oxidation States:
The oxidation state, or oxidation number, represents the apparent charge of an atom in a compound. Elements within the same group often display similar oxidation states, reflecting their tendency to gain or lose a consistent number of electrons during bonding. This consistency is again directly linked to their similar valence electron configurations. For example, Group 1 elements typically exhibit a +1 oxidation state because they readily lose one electron.
3. Types of Bonds Formed:
The number of valence electrons influences the type of bonds an element will form. Elements with few valence electrons tend to lose electrons and form ionic bonds with elements that readily gain electrons. Elements with many valence electrons may share electrons to form covalent bonds. The consistency in valence electrons within a group means that elements in the same group will often form similar types of bonds with other elements.
Similar Physical Properties: Atomic Radius, Ionization Energy, and Electronegativity
Besides chemical properties, elements in the same group also exhibit striking similarities in certain physical properties:
1. Atomic Radius:
Atomic radius refers to the size of an atom. While atomic radius increases as you move down a group (due to the addition of electron shells), elements within the same group show a relatively consistent trend compared to elements across periods. This similarity in size contributes to similar physical properties such as density and melting point.
2. Ionization Energy:
Ionization energy is the energy required to remove an electron from an atom. Elements within the same group tend to exhibit similar ionization energies, although the energy generally decreases as you move down a group. This is because the valence electrons are further from the nucleus in larger atoms, making them easier to remove.
3. Electronegativity:
Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond. Electronegativity generally decreases as you move down a group, reflecting the increasing distance between the valence electrons and the nucleus. However, the relative electronegativity within a group remains more consistent than when comparing elements across periods.
Exceptions and Nuances: The Importance of Context
While the general trend of similar properties within groups holds true, it's crucial to acknowledge exceptions and nuances. The periodic table isn't a rigid, unwavering rulebook; rather, it's a framework for understanding trends and predicting behavior. Some factors that can influence these trends include:
-
Relativistic Effects: In heavier elements, relativistic effects (changes in electron behavior due to high speeds) can alter properties in unexpected ways. This is particularly noticeable in the later transition metal groups.
-
Lanthanide and Actinide Contraction: The poor shielding effect of the f-electrons in the lanthanides and actinides causes a decrease in atomic radius, impacting properties of elements in subsequent groups.
-
Anomalous Behavior of Certain Elements: Certain elements may show deviations from the expected trends due to specific electronic configurations or other factors. For example, some transition metals exhibit multiple oxidation states, complicating the prediction of their behavior solely based on group placement.
Applications and Importance: Predicting and Understanding Chemical Behavior
The understanding that elements in the same group have similar properties is fundamental to numerous applications in chemistry and other scientific fields.
1. Predicting Chemical Reactions:
Chemists can leverage this understanding to predict the outcome of chemical reactions. Knowing that elements in a specific group typically react in a certain way allows for the prediction of reaction products and the design of experiments.
2. Material Science:
This knowledge is essential in the development of new materials. By understanding the properties of elements within a particular group, researchers can create materials with specific desired characteristics, such as conductivity, strength, or reactivity.
3. Environmental Science:
Understanding the chemical behavior of elements helps in assessing environmental impacts. The similarities in properties within a group allow scientists to predict the behavior of pollutants and develop effective remediation strategies.
4. Medical Applications:
In medicine, the predictable properties of elements within groups are crucial in developing drugs and understanding biological processes. For example, the similarities in reactivity between elements in a group can be exploited to design drugs with specific targets.
Conclusion: A Powerful Tool for Chemical Prediction
The observation that elements in the same group have similar properties is a cornerstone of modern chemistry. This similarity, rooted in the identical number of valence electrons, allows for the prediction of chemical and physical behavior, leading to significant advancements in various fields. While exceptions and nuances exist, the overall trend remains remarkably consistent and provides a powerful tool for understanding and manipulating the behavior of matter. Further research continues to refine our understanding of these trends, revealing more intricate details and expanding the applications of this fundamental principle. The periodic table, therefore, serves not just as an organizational chart but as a predictive model, guiding chemical research and innovation.
Latest Posts
Latest Posts
-
How To Find The Mass Of The Excess Reactant
Apr 02, 2025
-
Instantaneous Rate Of Change Vs Average Rate Of Change
Apr 02, 2025
-
How Many D Orbitals Can Be In An Energy Level
Apr 02, 2025
-
Log Base 2 X 2 Graph
Apr 02, 2025
-
X 3 2x 2 5x 6
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Elements In The Same Group Have Similar . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
