Which State Of Matter Has The Highest Kinetic Energy
listenit
Mar 25, 2025 · 5 min read
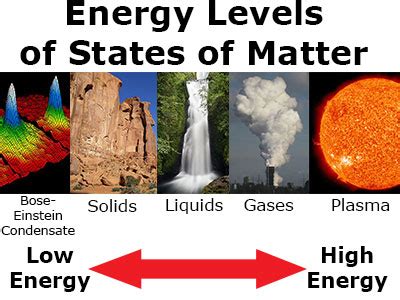
Table of Contents
Which State of Matter Has the Highest Kinetic Energy?
Understanding the relationship between kinetic energy and the states of matter (solid, liquid, gas, and plasma) is crucial to grasping fundamental concepts in physics and chemistry. While a simple answer might seem readily available, the reality is more nuanced and depends on several factors, making this question a rich area for exploration. This article delves deep into the kinetic energy of different states of matter, exploring the complexities and subtleties involved.
Defining Kinetic Energy and its Relation to Temperature
Before diving into the different states, let's establish a clear definition. Kinetic energy is the energy an object possesses due to its motion. The faster an object moves, the greater its kinetic energy. At the microscopic level, this translates to the movement of atoms and molecules within a substance. Temperature, a macroscopic property, is directly proportional to the average kinetic energy of these particles. Higher temperature signifies greater average kinetic energy.
The Role of Intermolecular Forces
The relationship between kinetic energy and the state of matter isn't solely determined by temperature. Intermolecular forces, the attractive forces between molecules, play a significant role. These forces vary in strength depending on the type of molecule and influence how freely the particles can move.
States of Matter and their Kinetic Energy
Let's examine each state of matter individually, analyzing their typical kinetic energy levels:
Solids: Low Kinetic Energy, Strong Intermolecular Forces
In solids, the constituent particles (atoms, ions, or molecules) are tightly packed together in a fixed arrangement. Their kinetic energy is relatively low. The strong intermolecular forces restrict the particles' movement to primarily vibrational motion around fixed points. While particles do vibrate, their movement is constrained, limiting their overall kinetic energy compared to other states. Think of the atoms in a solid crystal lattice – they jiggle in place but don't move freely. Therefore, solids generally exhibit the lowest average kinetic energy at a given temperature.
Liquids: Moderate Kinetic Energy, Weaker Intermolecular Forces
In liquids, the intermolecular forces are weaker than in solids, allowing the particles more freedom of movement. They can move past one another, leading to fluidity. The kinetic energy is higher than in solids, resulting in a less structured arrangement and greater particle mobility. The particles still interact significantly, preventing them from flying apart like in a gas. Therefore, liquids have a moderate average kinetic energy, significantly higher than solids at the same temperature.
Gases: High Kinetic Energy, Negligible Intermolecular Forces
Gases exhibit the highest kinetic energy among the three common states of matter (solid, liquid, gas). The intermolecular forces are negligible compared to the kinetic energy of the particles. This allows the particles to move freely and randomly, frequently colliding with each other and the container walls. Their high kinetic energy results in a much larger volume compared to solids and liquids at the same temperature and pressure. This free movement and frequent collisions contribute to the expansive nature of gases.
Plasma: The Highest Kinetic Energy State
Plasma, often called the fourth state of matter, represents a distinct state with unique characteristics. It's an ionized gas where a significant fraction of the atoms or molecules have lost or gained electrons, resulting in the presence of freely moving ions and electrons. The kinetic energy in plasma is significantly higher than in gases, because it is characterized by extremely high temperatures and consequently high particle velocities. The strong electrostatic interactions between charged particles further contribute to the high kinetic energy. This high kinetic energy is responsible for plasma's unique properties, such as its ability to conduct electricity and its response to magnetic fields.
Factors Influencing Kinetic Energy Beyond State of Matter
While the state of matter provides a general indication of kinetic energy, other factors play a significant role:
-
Temperature: As mentioned earlier, temperature is directly proportional to average kinetic energy. A higher temperature means higher average kinetic energy, irrespective of the state of matter. However, the change in kinetic energy with temperature varies depending on the state. For example, a small temperature increase might drastically change the state of a solid close to its melting point, while it may have a less noticeable impact on a gas.
-
Mass of Particles: Heavier particles at the same temperature will possess lower average velocities than lighter particles. This is because kinetic energy is related to both mass and velocity (KE = 1/2mv²). Therefore, even in the same state, substances with different molecular weights will have different kinetic energy levels at the same temperature.
-
Pressure: Pressure significantly impacts the kinetic energy of gases. Higher pressure implies more frequent collisions, leading to increased average kinetic energy. This effect is less pronounced in liquids and solids due to the stronger intermolecular forces.
The Nuances and Exceptions
The general trend – solids < liquids < gases < plasma – in terms of kinetic energy holds true under typical conditions. However, exceptions exist. For example:
-
High-pressure liquids and solids: Under extreme pressure, the kinetic energy of solids and liquids can be altered, potentially becoming comparable to gases at lower pressures.
-
Low-temperature gases: At extremely low temperatures, the kinetic energy of a gas might drop drastically, causing it to behave more like a liquid or even solidify.
-
Specific substances: The properties of individual molecules and the strength of their intermolecular forces can significantly impact the kinetic energy at a specific temperature and pressure. Highly polar molecules, for instance, might have higher intermolecular forces and therefore lower kinetic energy compared to non-polar molecules in the same state and at the same temperature.
Conclusion: It's Not Always a Straightforward Answer
The question of which state of matter possesses the highest kinetic energy isn't always straightforward. While plasma generally exhibits the highest kinetic energy due to its highly energized particles and ionized state, the relationship is complex and depends on temperature, pressure, the mass of the particles involved, and the strength of intermolecular forces. Understanding these intricacies is vital for a comprehensive understanding of the behavior of matter in different states. Remember that temperature is the most significant factor, directly influencing the average kinetic energy of particles regardless of their state. However, the state of matter dictates the range and type of motion, which affects overall kinetic energy significantly.
Latest Posts
Latest Posts
-
How To Find Number Of Core Electrons
Mar 28, 2025
-
What Is The Formula For The Compound Magnesium Oxide
Mar 28, 2025
-
What Is The Correct Formula For Calcium Oxide
Mar 28, 2025
-
What Is The Si Base Unit Of Length
Mar 28, 2025
-
What Is The Oxidation State Of Each Element In Coh2
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Which State Of Matter Has The Highest Kinetic Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
