What Is The Correct Formula For Calcium Oxide
listenit
Mar 28, 2025 · 6 min read
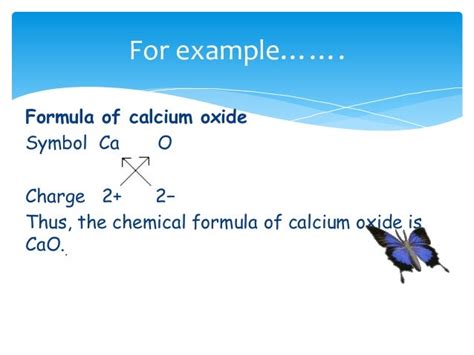
Table of Contents
What is the Correct Formula for Calcium Oxide? A Deep Dive into Chemical Composition and Properties
Calcium oxide, a ubiquitous compound in various industrial and natural processes, is a cornerstone of chemistry and material science. Understanding its correct formula is crucial for comprehending its reactions, applications, and overall importance. This article delves deep into the chemical composition of calcium oxide, exploring its formula, properties, production methods, and wide-ranging uses. We will also examine related compounds and potential misconceptions surrounding its formula.
The Definitive Formula: CaO
The correct chemical formula for calcium oxide is CaO. This simple yet powerful formula represents the precise stoichiometric ratio of calcium (Ca) and oxygen (O) atoms within the compound. One calcium atom bonds with one oxygen atom, forming a strong ionic bond due to the significant electronegativity difference between the two elements. Calcium, an alkaline earth metal, readily loses two electrons to achieve a stable octet, forming a Ca²⁺ cation. Oxygen, a highly electronegative nonmetal, gains these two electrons to become an O²⁻ anion. The electrostatic attraction between these oppositely charged ions results in the formation of the crystalline calcium oxide structure.
Understanding Ionic Bonding in CaO
The ionic bond in CaO is a primary driver of its properties. The strong electrostatic forces holding the Ca²⁺ and O²⁻ ions together lead to a high melting point (approximately 2572 °C or 4662 °F) and a crystalline structure. This robust bonding contributes to its stability and resistance to decomposition under normal conditions. The crystalline structure, typically cubic, influences its physical properties like hardness and refractivity.
Production Methods: From Limestone to Quicklime
Calcium oxide is primarily produced through the thermal decomposition of calcium carbonate (CaCO₃), commonly found in limestone and other carbonate-rich rocks. This process, known as calcination, involves heating the limestone to high temperatures (typically above 825 °C or 1517 °F) in a kiln. The reaction can be represented as:
CaCO₃(s) → CaO(s) + CO₂(g)
This reaction releases carbon dioxide gas (CO₂), leaving behind calcium oxide, also known as quicklime. The efficiency of the calcination process depends on several factors, including the temperature, residence time in the kiln, and the purity of the limestone. Modern kilns employ sophisticated techniques to optimize these parameters for maximum yield and energy efficiency. The process is crucial in the large-scale production of calcium oxide, which is used extensively in diverse industrial applications.
Purity and Impurities in Commercial Calcium Oxide
Commercial calcium oxide samples rarely exhibit 100% purity. Impurities commonly found include magnesium oxide (MgO), silica (SiO₂), iron oxides (Fe₂O₃), and alumina (Al₂O₃). These impurities are usually carried over from the limestone source material and can affect the properties and applications of the calcium oxide. The purity level is crucial for specific applications, demanding stringent control during the production and purification processes.
Diverse Applications of Calcium Oxide: A Multifaceted Compound
The wide-ranging applications of calcium oxide stem from its unique chemical and physical properties. Its reactivity with water, acids, and other substances, coupled with its high melting point and refractory nature, makes it a versatile material used in a broad spectrum of industries:
1. Construction and Building Materials:
- Cement Production: Calcium oxide is a key ingredient in Portland cement, a crucial component of concrete. Its reaction with water (hydration) is a fundamental step in the setting and hardening of cement. This reaction generates heat, making it an exothermic process that contributes to the strength of the concrete.
- Mortar and Plaster: Quicklime is used in the production of mortars and plasters, acting as a binding agent that holds bricks and other building materials together. Its ability to absorb moisture and carbon dioxide contributes to the durability and longevity of these constructions.
- Lime Stabilization: Adding calcium oxide to soil can improve its stability and reduce its plasticity, particularly useful in road construction and other geotechnical applications.
2. Industrial Processes:
- Steelmaking: Calcium oxide is a fluxing agent in steelmaking, removing impurities like silica and phosphorus from molten iron. This process is crucial for improving the quality and strength of the steel.
- Pulp and Paper Industry: Calcium oxide is used in the kraft pulping process to recover chemicals and improve the efficiency of paper production. It plays a crucial role in the recycling of spent pulping liquors.
- Water Treatment: Calcium oxide is used to adjust the pH of water and remove impurities, playing a vital role in the purification and softening of drinking water. Its reaction with acidic substances neutralizes the water's acidity.
3. Chemical Industry:
- Chemical Synthesis: Calcium oxide is a reactant in various chemical syntheses, producing other calcium compounds or participating in diverse chemical reactions. Its role as a base makes it vital in many industrial chemical processes.
- Catalyst and Catalyst Support: Calcium oxide can act as a catalyst or a support material for catalysts in several industrial chemical reactions, influencing the reaction rate and selectivity.
4. Other Applications:
- Agriculture: Calcium oxide can be used to improve soil pH and nutrient availability, benefitting crop growth and yield. It helps to neutralize acidic soil conditions, making it more suitable for plant cultivation.
- Food Industry: In certain food processing applications, calcium oxide can act as a food additive for specific purposes such as a pH adjuster or a firming agent. However, regulations and permissible levels vary across jurisdictions.
- Environmental Remediation: Calcium oxide has been explored for its potential in various environmental remediation applications, including neutralizing acidic waste streams or stabilizing hazardous materials.
Related Compounds and Potential Misconceptions
Understanding the formula for calcium oxide also requires familiarity with related calcium compounds which could potentially cause confusion:
- Calcium hydroxide (Ca(OH)₂): This compound, also known as slaked lime, is formed when calcium oxide reacts with water. This reaction is highly exothermic and releases significant heat. It's crucial to differentiate between CaO and Ca(OH)₂, as they possess different properties and applications.
- Calcium carbonate (CaCO₃): As mentioned earlier, this is the primary source material for calcium oxide production. It's important not to confuse the starting material with the final product.
A common misconception is that the formula might include water molecules. Anhydrous calcium oxide, denoted by CaO, does not contain water. However, exposure to atmospheric moisture can lead to the formation of calcium hydroxide, which incorporates water molecules into its structure.
Conclusion: The Importance of Accurate Chemical Formulae
The correct formula for calcium oxide, CaO, is fundamental to understanding its chemical behavior, properties, and applications. This simple formula encapsulates the strong ionic bond between calcium and oxygen, the basis for its diverse roles in construction, industry, and other fields. Accurate chemical formulae are essential for precise chemical calculations, safe handling, and effective application of chemical compounds in various industries and scientific endeavors. This deep dive into the chemical composition, production, and uses of calcium oxide highlights its importance and the significance of understanding its correct chemical formula. The formula provides a concise yet comprehensive representation of a compound's elemental composition and is crucial for precise chemical calculations, understanding reactions, and safe handling. Misunderstanding the formula can lead to inaccurate predictions, ineffective applications, and even safety hazards.
Latest Posts
Latest Posts
-
What Type Of Medium Travels The Fastest
Mar 31, 2025
-
1 3 To The Power Of 3
Mar 31, 2025
-
Least Common Multiple For 18 And 24
Mar 31, 2025
-
What Is 3 4 Equivalent To In Fractions
Mar 31, 2025
-
Where Is Most Freshwater Found On Earth
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is The Correct Formula For Calcium Oxide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
