How To Find Number Of Core Electrons
listenit
Mar 28, 2025 · 6 min read
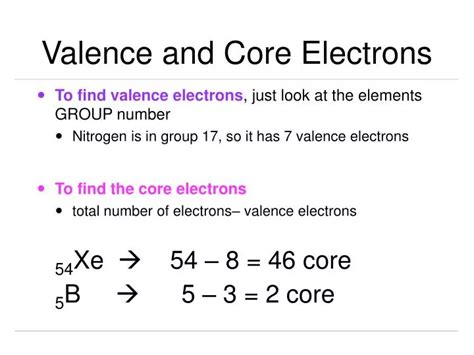
Table of Contents
How to Find the Number of Core Electrons: A Comprehensive Guide
Determining the number of core electrons in an atom is a fundamental concept in chemistry. Understanding this allows us to predict an element's chemical behavior and properties. While seemingly straightforward, the process involves a nuanced understanding of electron configuration and atomic structure. This comprehensive guide will walk you through various methods for calculating the number of core electrons, catering to different levels of understanding.
Understanding Atomic Structure and Electron Configuration
Before diving into the methods, let's refresh our understanding of key concepts:
-
Atomic Number (Z): This represents the number of protons in an atom's nucleus, which is also equal to the number of electrons in a neutral atom. This is a crucial piece of information for determining core electrons.
-
Electron Shells (Energy Levels): Electrons reside in shells or energy levels surrounding the nucleus. These shells are numbered sequentially (n=1, n=2, n=3, and so on), with n=1 being the closest to the nucleus.
-
Electron Subshells (Sublevels): Each shell contains subshells (s, p, d, f) with varying energy levels and capacities. The 's' subshell holds a maximum of 2 electrons, 'p' holds 6, 'd' holds 10, and 'f' holds 14.
-
Electron Configuration: This notation describes the arrangement of electrons within an atom's subshells. For example, the electron configuration of oxygen (atomic number 8) is 1s²2s²2p⁴.
-
Valence Electrons: These are the outermost electrons in the highest energy level. They participate in chemical bonding.
-
Core Electrons: These are the electrons that are not valence electrons. They occupy the inner energy levels and are tightly bound to the nucleus. They are essentially "core" to the atom's structure and are less involved in chemical reactions.
Methods for Determining the Number of Core Electrons
There are several methods to determine the number of core electrons, each with varying levels of complexity:
Method 1: Using the Atomic Number and the Noble Gas Configuration
This is the most efficient method for most atoms. It leverages the periodic table's organization and the noble gas configuration.
-
Locate the Element: Find the element on the periodic table using its atomic number or symbol.
-
Identify the Noble Gas: Find the noble gas that precedes the element in the same period (row). Noble gases have filled electron shells (stable octet).
-
Determine the Number of Valence Electrons: Count the number of electrons the element has beyond the noble gas configuration. This can be found by subtracting the atomic number of the noble gas from the atomic number of the element. This number represents the valence electrons.
-
Calculate Core Electrons: Subtract the number of valence electrons from the total number of electrons (atomic number). The result is the number of core electrons.
Example: Let's find the number of core electrons in Chlorine (Cl), atomic number 17.
-
The noble gas preceding Chlorine is Neon (Ne), atomic number 10.
-
Chlorine has 17 - 10 = 7 valence electrons.
-
Therefore, Chlorine has 17 - 7 = 10 core electrons.
This method directly utilizes the periodic table's structure, making it intuitive and quick.
Method 2: Using the Electron Configuration
This method involves writing out the full electron configuration and then counting the electrons not in the outermost shell.
-
Write the Electron Configuration: Determine the complete electron configuration of the element. You can do this by following the Aufbau principle and Hund's rule. Remember to fill subshells in order of increasing energy.
-
Identify the Outermost Shell: Determine the highest principal quantum number (n) in the electron configuration. This is the outermost shell.
-
Count Core Electrons: Count all electrons below the outermost shell. These are the core electrons.
Example: Let's again consider Chlorine (Cl), atomic number 17.
-
The electron configuration of Chlorine is 1s²2s²2p⁶3s²3p⁵.
-
The outermost shell is n=3.
-
The core electrons are in shells n=1 and n=2: 2 (1s²) + 2 (2s²) + 6 (2p²) = 10 core electrons.
This method is more involved, but it provides a deeper understanding of electron distribution. It's helpful for reinforcing the principles of electron configuration.
Method 3: Using the Period Number (Less Reliable)
This method provides a rough estimate, especially for elements in the later periods. It's not as accurate as the previous two methods, especially for transition metals and inner transition metals (lanthanides and actinides).
-
Locate the Element: Find the element on the periodic table.
-
Identify the Period: Determine the period (row) number of the element.
-
Estimate Core Electrons: For elements in the first three periods, the period number approximately indicates the number of shells containing core electrons. The number of core electrons is roughly 2*(period number -1) for elements not in the d or f block.
Example (Approximate): For Chlorine (Period 3), this method would suggest roughly 2 * (3-1) = 4 core electrons. This is a significant underestimation compared to the actual value of 10.
Why this method is less reliable: This method doesn't account for the nuances of electron shell filling, particularly for transition metals and inner transition metals where the d and f subshells are filled. This results in a highly inaccurate estimation of core electrons for these elements.
Important Considerations and Advanced Topics
-
Ions: For ions (charged atoms), the number of electrons changes. Remember to adjust the number of electrons based on the charge. For example, a Cl⁻ ion (chloride) has one more electron (18) than a neutral chlorine atom.
-
Transition Metals: Determining core electrons for transition metals requires careful consideration of the filling of the d subshells. The outermost electrons are often considered as d-electrons rather than s-electrons, especially in certain oxidation states.
-
Inner Transition Metals (Lanthanides and Actinides): The f subshells add further complexity. The exact number of core electrons needs precise analysis of the electron configuration.
-
Exceptions to the Aufbau Principle: Certain elements exhibit deviations from the standard Aufbau principle (the order of subshell filling). For these exceptions, the actual electron configuration must be used to accurately determine the number of core electrons.
Conclusion: Mastering Core Electron Calculation
Determining the number of core electrons is crucial for understanding atomic structure and chemical behavior. While the method using noble gas configurations is the most efficient and accurate for most elements, understanding the electron configuration method provides a deeper insight into electron distribution. Remember that the simplified method using period numbers is not suitable for most cases and should be avoided for accurate results, especially for transition and inner transition metals. By mastering these techniques and considering the special cases, you will develop a strong foundation in fundamental chemistry. Remember to always consult a periodic table and accurate electron configuration resources to ensure accuracy in your calculations.
Latest Posts
Latest Posts
-
What Is 3 4 Equivalent To In Fractions
Mar 31, 2025
-
Where Is Most Freshwater Found On Earth
Mar 31, 2025
-
Simplify The Square Root Of 512
Mar 31, 2025
-
2 1 6 As An Improper Fraction
Mar 31, 2025
-
Explain One Major Difference Between Purines And Pyrimidines
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How To Find Number Of Core Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
