Which Particle Is Found In The Nucleus Of The Atom
listenit
Mar 17, 2025 · 6 min read
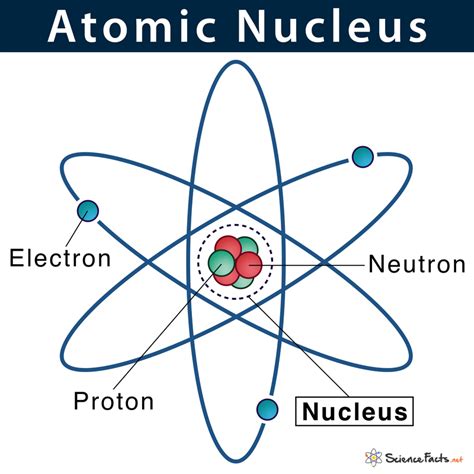
Table of Contents
Which Particle is Found in the Nucleus of the Atom? A Deep Dive into Atomic Structure
The atom, the fundamental building block of matter, is a fascinating world of subatomic particles. Understanding its structure is key to grasping the principles of chemistry, physics, and numerous other scientific disciplines. This article will delve into the specifics of the atom's nucleus, focusing on the particles that reside within, exploring their properties and their roles in determining an atom's characteristics.
The Nucleus: The Atom's Core
At the heart of every atom lies its nucleus, a tiny, dense region containing the majority of the atom's mass. This nucleus is not a simple entity; it's a complex system of particles held together by incredibly powerful forces. The primary particles found within the nucleus are protons and neutrons.
Protons: Positively Charged Powerhouses
Protons are positively charged subatomic particles. Their charge is equal in magnitude but opposite in sign to the electron's negative charge. This positive charge is crucial because it dictates the atom's chemical properties and its interactions with other atoms. The number of protons in an atom's nucleus defines its atomic number, a fundamental characteristic that uniquely identifies each element on the periodic table. For example, hydrogen (H) has one proton, helium (He) has two, and oxygen (O) has eight.
Key properties of protons:
- Charge: +1 (elementary charge)
- Mass: Approximately 1.6726 x 10<sup>-27</sup> kg (approximately 1836 times the mass of an electron)
- Spin: 1/2 (a fundamental quantum property)
- Symbol: p or p<sup>+</sup>
Neutrons: Neutral Mass Contributors
Neutrons are neutral particles, meaning they carry no electric charge. While they don't directly influence an atom's chemical behavior in the same way protons do, their presence significantly impacts the atom's mass and stability. The number of neutrons in an atom's nucleus, combined with the number of protons, determines the atom's mass number. Isotopes of the same element have the same number of protons but differ in their number of neutrons.
Key properties of neutrons:
- Charge: 0
- Mass: Approximately 1.6749 x 10<sup>-27</sup> kg (slightly larger than the mass of a proton)
- Spin: 1/2
- Symbol: n
The Strong Nuclear Force: The Glue that Holds the Nucleus Together
The protons within the nucleus all possess a positive charge, and like charges repel each other. Therefore, a powerful force is needed to overcome this electrostatic repulsion and bind the protons together. This force is known as the strong nuclear force, one of the four fundamental forces in nature. It is much stronger than the electromagnetic force at short distances, but its influence diminishes rapidly with increasing distance. The strong nuclear force also acts on neutrons, contributing to the overall stability of the nucleus.
The balance between the strong nuclear force and the electromagnetic repulsion between protons is critical for nuclear stability. Too few neutrons, and the electromagnetic repulsion can overcome the strong force, leading to nuclear instability and radioactive decay. Conversely, too many neutrons can also destabilize the nucleus. The optimal neutron-to-proton ratio varies depending on the element.
Isotopes: Variations on a Theme
As mentioned earlier, isotopes are atoms of the same element (same number of protons) that differ in their number of neutrons. This difference in neutron number leads to variations in mass number and, in some cases, nuclear stability. Some isotopes are stable, while others are radioactive, meaning their nuclei decay spontaneously, emitting particles or energy. Radioactive isotopes have numerous applications in various fields, including medicine, archaeology, and industry.
Beyond Protons and Neutrons: Exploring the Quark Model
While protons and neutrons were once considered fundamental particles, further research revealed a deeper level of structure. Both protons and neutrons are composed of smaller particles called quarks. Specifically, protons are made up of two up quarks and one down quark, while neutrons consist of one up quark and two down quarks. Quarks are held together by the strong force, mediated by particles called gluons.
Quarks: Fundamental Building Blocks
Quarks are fundamental particles, meaning they are not composed of smaller constituents (as far as we currently know). They possess fractional electric charges: up quarks have a charge of +2/3, and down quarks have a charge of -1/3. The combination of these charges in protons and neutrons results in their overall charges of +1 and 0, respectively.
Several types of quarks exist, but up and down quarks are the most common and are the constituents of protons and neutrons. Other types of quarks include charm, strange, top, and bottom quarks. These heavier quarks are typically found in short-lived, high-energy particles.
Gluons: The Mediators of the Strong Force
Gluons are the force-carrying particles responsible for the strong force that binds quarks together within protons and neutrons. They are massless and have no electric charge. Unlike photons (the force-carrying particles of the electromagnetic force), gluons themselves carry the strong force charge, leading to a complex interaction that contributes to the confinement of quarks within protons and neutrons. It's not possible to isolate a single quark because the strong force increases with distance, making it incredibly difficult to separate them.
Nuclear Reactions: Transformations in the Nucleus
The nucleus is not immutable. Under certain conditions, such as high energy collisions or radioactive decay, the composition of the nucleus can change. These changes are known as nuclear reactions. These reactions involve the transformation of protons and neutrons, potentially leading to the formation of new elements or isotopes.
Conclusion: The Nucleus – A Dynamic and Crucial Part of the Atom
The nucleus of the atom, containing protons and neutrons (which are themselves composed of quarks), is a dynamic and crucial component determining an element's identity and properties. Its stability, influenced by the strong nuclear force and the balance of protons and neutrons, dictates whether an atom will be stable or radioactive. Understanding the intricacies of the nucleus is essential to comprehending a wide range of phenomena in physics, chemistry, and beyond. From the energy harnessed in nuclear power plants to the medical applications of radioactive isotopes, the study of the atomic nucleus continues to be a vibrant and important field of scientific research. The exploration into the subatomic realm, including the intricacies of quarks and gluons, reveals ever-increasing layers of complexity and fundamental understanding about the universe around us. The simple answer to the question "Which particle is found in the nucleus of the atom?" is thus far more complex and fascinating than it initially appears.
Latest Posts
Latest Posts
-
How Many Degrees Celsius Is 1 Degrees Fahrenheit
Mar 17, 2025
-
How Do You Find Heat Energy That Water Gains
Mar 17, 2025
-
4x 2y 12 4x 8y 24
Mar 17, 2025
-
What Is The Lowest Point Of A Wave Called
Mar 17, 2025
-
Can Acids Or Bases Conduct Electricity
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Which Particle Is Found In The Nucleus Of The Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
