Can Acids Or Bases Conduct Electricity
listenit
Mar 17, 2025 · 5 min read
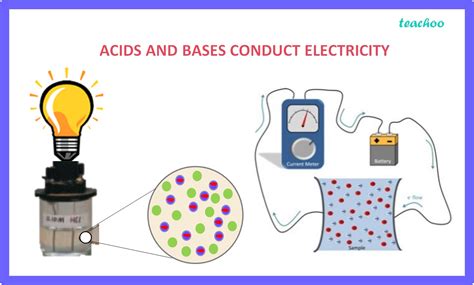
Table of Contents
Can Acids or Bases Conduct Electricity? Understanding Electrolytes and Ionic Solutions
The ability of a substance to conduct electricity hinges on its capacity to allow the free movement of charged particles, known as ions. Pure water, for example, is a poor conductor because it has a very low concentration of ions. However, when acids or bases are dissolved in water, they dramatically increase the concentration of ions, transforming the solution into a good conductor of electricity. This article delves into the reasons behind this phenomenon, exploring the behavior of acids and bases in solution and their impact on electrical conductivity.
The Role of Ions in Electrical Conductivity
Electrical conductivity is essentially the measure of a substance's ability to allow the flow of electric current. This flow is facilitated by the movement of charged particles, specifically ions – atoms or molecules that have gained or lost electrons, carrying a net positive or negative charge. In solids, like metals, this conductivity is primarily due to the free movement of electrons. However, in liquids, it's the movement of ions that determines the conductivity.
Acids and bases, when dissolved in water, undergo a process called ionization or dissociation. This process releases ions into the solution, significantly increasing its conductivity. The greater the concentration of ions, the higher the conductivity of the solution.
Acids: Ionization and Conductivity
Acids are substances that donate protons (H⁺ ions) when dissolved in water. This donation process leads to the formation of hydronium ions (H₃O⁺), which are positively charged, and anionic species, which are negatively charged. The strength of an acid determines the extent of ionization.
Strong Acids: Complete Ionization
Strong acids, such as hydrochloric acid (HCl), sulfuric acid (H₂SO₄), and nitric acid (HNO₃), completely ionize in water. This means that virtually every acid molecule dissociates into its constituent ions. For example, HCl dissociates as follows:
HCl(aq) → H⁺(aq) + Cl⁻(aq)
The resulting solution contains a high concentration of H₃O⁺ and Cl⁻ ions, making it an excellent conductor of electricity. The higher the concentration of the strong acid, the greater the conductivity.
Weak Acids: Partial Ionization
Weak acids, such as acetic acid (CH₃COOH) and carbonic acid (H₂CO₃), only partially ionize in water. This means that only a small fraction of the acid molecules dissociate into ions. The equilibrium lies far to the left, meaning a significant amount of undissociated acid molecules remain. For example, the ionization of acetic acid is represented as:
CH₃COOH(aq) ⇌ CH₃COO⁻(aq) + H⁺(aq)
Because of the limited ionization, weak acid solutions have lower conductivity compared to strong acid solutions of similar concentrations. The conductivity of a weak acid solution is directly related to the degree of ionization, which is influenced by factors like temperature and concentration.
Bases: Dissociation and Conductivity
Bases are substances that accept protons (H⁺ ions) or donate hydroxide ions (OH⁻) when dissolved in water. Similar to acids, the strength of a base influences its degree of dissociation and consequently, its conductivity.
Strong Bases: Complete Dissociation
Strong bases, like sodium hydroxide (NaOH) and potassium hydroxide (KOH), completely dissociate in water, releasing a high concentration of hydroxide ions (OH⁻) and metal cations (Na⁺ or K⁺). For instance, the dissociation of NaOH is:
NaOH(aq) → Na⁺(aq) + OH⁻(aq)
These solutions are excellent conductors of electricity due to the abundance of ions.
Weak Bases: Partial Dissociation
Weak bases, like ammonia (NH₃), partially dissociate in water. They react with water to form hydroxide ions and the conjugate acid. For example, the dissociation of ammonia is:
NH₃(aq) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
The equilibrium favors the reactants, meaning only a small fraction of ammonia molecules form hydroxide ions. As a result, weak base solutions have lower electrical conductivity compared to strong bases of similar concentrations.
Factors Affecting Conductivity of Acid and Base Solutions
Several factors influence the conductivity of acid and base solutions:
- Concentration: Higher concentrations of acids and bases lead to higher conductivity because more ions are present in the solution.
- Temperature: Increased temperature generally increases the conductivity of both acid and base solutions. This is because higher temperatures lead to increased molecular motion, facilitating ion movement.
- Strength of the acid/base: Strong acids and bases have higher conductivity than weak acids and bases due to their greater degree of ionization.
- Solvent: The nature of the solvent also plays a role. Water is a common solvent, but other solvents can affect the ionization and thus the conductivity of acids and bases.
- Presence of other ions: The presence of other dissolved salts can increase the overall ionic strength of the solution and thus impact conductivity.
Experimental Determination of Conductivity
The conductivity of acid and base solutions can be measured experimentally using a conductivity meter. This device measures the ability of a solution to conduct an electric current. The conductivity is usually expressed in Siemens per meter (S/m) or millisiemens per centimeter (mS/cm).
Applications of Electrical Conductivity Measurement
The measurement of electrical conductivity finds numerous applications in various fields:
- Monitoring water quality: Conductivity measurements are crucial in assessing the purity of water, detecting dissolved impurities, and monitoring pollution levels.
- Industrial processes: Conductivity monitoring is essential in many industrial processes, such as chemical manufacturing, electroplating, and wastewater treatment.
- Soil science: Soil conductivity provides insights into soil salinity, moisture content, and nutrient availability.
- Medical applications: Conductivity measurements are used in various medical diagnostic tools and procedures.
Conclusion: Conductivity and the Ionic Nature of Acids and Bases
The electrical conductivity of acid and base solutions is a direct consequence of the presence of ions in the solution. Strong acids and bases, which completely ionize or dissociate in water, exhibit high conductivity. Weak acids and bases, which partially ionize, exhibit lower conductivity. The concentration, temperature, and strength of the acid or base, as well as the presence of other ions and the nature of the solvent, all influence the conductivity of the solution. Understanding these relationships is vital in numerous scientific and industrial applications. Further research into specific acids and bases and their behavior under different conditions allows for a deeper understanding of electrolyte solutions and their crucial role in various processes. The measurement of conductivity provides valuable insights into the concentration and nature of ions within a solution, enabling accurate assessment and control in various practical applications.
Latest Posts
Latest Posts
-
How Many Protons And Neutrons Does Chlorine Have
May 09, 2025
-
How Do You Find The Perimeter Of A Equilateral Triangle
May 09, 2025
-
500 Ml Is What In Ounces
May 09, 2025
-
What Do All Of The Inner Planets Have In Common
May 09, 2025
-
Do Protists Make Their Own Food
May 09, 2025
Related Post
Thank you for visiting our website which covers about Can Acids Or Bases Conduct Electricity . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.