How Do You Find Heat Energy That Water Gains
listenit
Mar 17, 2025 · 5 min read
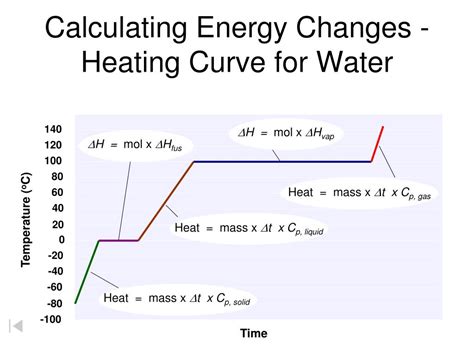
Table of Contents
How Do You Find the Heat Energy That Water Gains?
Determining the heat energy gained by water is a fundamental concept in thermodynamics with applications spanning various fields, from everyday cooking to sophisticated industrial processes. Understanding how to calculate this energy transfer is crucial for anyone working with heat, temperature changes, and water. This comprehensive guide will explore the various methods and factors involved in accurately determining the heat energy gained by water.
Understanding Specific Heat Capacity
At the heart of calculating heat energy gain in water lies the concept of specific heat capacity. This crucial property represents the amount of heat energy required to raise the temperature of one gram (or one kilogram) of a substance by one degree Celsius (or one Kelvin). Water possesses a remarkably high specific heat capacity compared to many other substances. This means it takes a significant amount of energy to change its temperature, a property that has profound implications for climate regulation and biological systems.
The specific heat capacity of water is approximately 4.186 joules per gram per degree Celsius (J/g°C) or 4186 joules per kilogram per degree Celsius (J/kg°C). This value is often denoted by the symbol 'c' in equations.
The Formula: Calculating Heat Energy Gain (Q)
The fundamental equation used to calculate the heat energy (Q) gained or lost by a substance, including water, is:
Q = mcΔT
Where:
- Q represents the heat energy gained or lost (in Joules, J)
- m represents the mass of the water (in grams, g, or kilograms, kg)
- c represents the specific heat capacity of water (4.186 J/g°C or 4186 J/kg°C)
- ΔT represents the change in temperature (in degrees Celsius, °C, or Kelvin, K). This is calculated as the final temperature (T<sub>f</sub>) minus the initial temperature (T<sub>i</sub>): ΔT = T<sub>f</sub> - T<sub>i</sub>
It's crucial to maintain consistent units throughout the calculation. If you use grams for mass, use the specific heat capacity in J/g°C. Similarly, if you use kilograms for mass, use the specific heat capacity in J/kg°C.
Step-by-Step Calculation
Let's illustrate the calculation with an example. Suppose we heat 200 grams of water from 20°C to 80°C. To find the heat energy gained (Q):
-
Identify the known variables:
- m = 200 g
- c = 4.186 J/g°C
- T<sub>i</sub> = 20°C
- T<sub>f</sub> = 80°C
-
Calculate the change in temperature (ΔT):
- ΔT = T<sub>f</sub> - T<sub>i</sub> = 80°C - 20°C = 60°C
-
Apply the formula:
- Q = mcΔT = (200 g) * (4.186 J/g°C) * (60°C) = 50232 J
Therefore, the water gained 50,232 Joules of heat energy.
Factors Affecting Heat Energy Gain
Several factors can influence the accuracy of the heat energy calculation:
1. Heat Loss to the Surroundings:
In reality, some heat energy is usually lost to the surroundings during the heating process (e.g., to the container, the air). This heat loss reduces the actual amount of heat energy absorbed by the water. To minimize this, well-insulated containers and controlled experimental conditions are necessary for more accurate measurements. Calorimetry experiments often employ techniques to account for or minimize heat loss.
2. Phase Changes:
The equation Q = mcΔT only applies when the water remains in the same phase (liquid). If the water undergoes a phase change (e.g., from liquid to gas during boiling), additional heat energy is required, and a different calculation involving latent heat of vaporization is needed.
3. Heat Sources and Efficiency:
The type and efficiency of the heat source used will also affect the overall heat transfer. Some heat sources are more efficient in transferring heat energy to the water than others.
4. Specific Heat Capacity Variations:
While the specific heat capacity of water is relatively constant over a wide temperature range, slight variations can occur at extreme temperatures or under specific conditions. For most practical calculations, the standard value (4.186 J/g°C) is sufficiently accurate.
Advanced Techniques and Considerations
For more precise measurements and in specific applications, more sophisticated techniques are often employed:
1. Calorimetry:
Calorimetry involves using a calorimeter, a device designed to minimize heat loss to the surroundings and accurately measure the heat energy exchanged during a process. Different types of calorimeters exist, each with its own design and capabilities.
2. Differential Scanning Calorimetry (DSC):
DSC is a thermoanalytic technique used to measure the heat flow associated with transitions in materials as a function of temperature. It can provide detailed information about phase transitions and other thermal events, which is particularly relevant when dealing with complex systems or phase changes.
3. Computational Fluid Dynamics (CFD):
For complex systems involving fluid flow and heat transfer, CFD simulations can provide a powerful way to model and predict heat energy gain in water. CFD models can account for factors such as fluid velocity, turbulence, and heat transfer mechanisms that are difficult to measure experimentally.
Applications of Heat Energy Calculations
The ability to accurately determine the heat energy gained by water has numerous applications across various disciplines:
- Engineering: Designing and optimizing heating and cooling systems, analyzing thermal performance of equipment, and understanding energy efficiency.
- Chemistry: Studying reaction kinetics, determining enthalpy changes in chemical reactions, and carrying out calorimetric experiments.
- Food Science: Understanding heat transfer during cooking processes, developing efficient food processing techniques, and ensuring food safety.
- Environmental Science: Analyzing heat transfer in aquatic ecosystems, studying climate change impacts on water bodies, and modeling water temperature dynamics.
- Meteorology: Predicting weather patterns, understanding the role of water in climate regulation, and forecasting extreme weather events.
- Medical Science: Designing and evaluating medical devices involving temperature control, studying biological processes involving heat transfer, and developing therapeutic applications.
Conclusion
Determining the heat energy gained by water is a fundamental calculation with significant practical implications. Understanding the formula Q = mcΔT, considering factors like heat loss and phase changes, and employing advanced techniques like calorimetry when necessary are all crucial for accurate results. The ability to perform these calculations is essential across various fields, contributing to advancements in engineering, science, and technology. As our understanding of thermal processes continues to grow, the importance of precise heat energy calculations will only increase. From simple experiments to complex simulations, mastery of this concept unlocks a deeper understanding of the world around us.
Latest Posts
Latest Posts
-
How Do You Find The Side Length Of A Square
May 09, 2025
-
Will Crayons Melt In The Car
May 09, 2025
-
Does Mass Affect Amplitude Of A Spring
May 09, 2025
-
48 As A Fraction In Simplest Form
May 09, 2025
-
A Strong Covalent Bond Between Adjacent Nucleotides Is
May 09, 2025
Related Post
Thank you for visiting our website which covers about How Do You Find Heat Energy That Water Gains . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.