Which Of The Following Elements Has The Largest Atomic Radius
listenit
Mar 25, 2025 · 5 min read
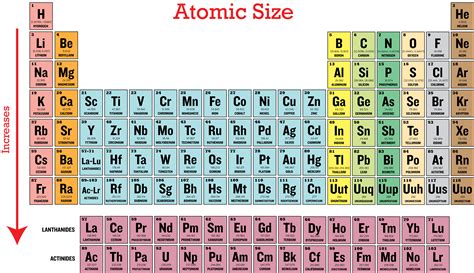
Table of Contents
Which of the Following Elements Has the Largest Atomic Radius? Understanding Atomic Size Trends
Determining which element possesses the largest atomic radius requires a deep understanding of atomic structure and the periodic trends that influence it. While a simple comparison of a few elements might seem straightforward, the nuances of electron shielding, effective nuclear charge, and electron-electron repulsion play a crucial role. This article will delve into these factors, explaining how they affect atomic size and ultimately allowing us to predict which element among a given set will exhibit the largest atomic radius.
Understanding Atomic Radius
The atomic radius, a measure of the size of an atom, isn't a fixed value. Its determination is complex and often depends on the method used. Commonly, we discuss covalent radius (half the distance between the nuclei of two identical atoms bonded together) or metallic radius (half the distance between adjacent nuclei in a metallic crystal). While the precise value fluctuates, the relative sizes of atoms across the periodic table remain consistent. Understanding this consistency is key to answering our central question.
Factors Influencing Atomic Radius
Several key factors influence an atom's size:
-
Principal Quantum Number (n): As we move down a group in the periodic table, the principal quantum number increases. This signifies the addition of electron shells. Each new shell is further from the nucleus, leading to a larger atomic radius. The higher the value of 'n', the greater the distance of the outermost electrons from the nucleus.
-
Effective Nuclear Charge (Z<sub>eff</sub>): This represents the net positive charge experienced by the outermost electrons. While the actual nuclear charge (number of protons) increases across a period, the increasing number of inner electrons effectively shields the outer electrons from the full positive charge of the nucleus. This shielding effect reduces the effective nuclear charge experienced by the valence electrons. A lower Z<sub>eff</sub> results in weaker attraction to the nucleus and a larger atomic radius.
-
Electron-Electron Repulsion: As the number of electrons in a shell increases, the repulsion between them becomes more significant. This repulsion pushes the electrons further apart, contributing to a larger atomic radius. This effect is particularly noticeable in larger atoms with multiple electrons in the outer shell.
Periodic Trends in Atomic Radius
These factors combine to create predictable trends in atomic radius across the periodic table:
-
Down a Group (Column): Atomic radius generally increases as you move down a group. The addition of electron shells significantly outweighs the increase in nuclear charge, leading to a larger atom.
-
Across a Period (Row): Atomic radius generally decreases as you move across a period from left to right. The increasing nuclear charge (more protons) pulls the electrons closer to the nucleus, despite the addition of electrons to the same shell. The increase in effective nuclear charge dominates the effect of electron-electron repulsion.
Comparing Atomic Radii: A Case Study
Let's consider a hypothetical scenario: We need to determine which of the following elements has the largest atomic radius: Sodium (Na), Potassium (K), Chlorine (Cl), and Argon (Ar).
-
Group Comparison: Sodium (Na) and Potassium (K) belong to Group 1 (alkali metals). Potassium (K) is below Sodium (Na), meaning it has an additional electron shell. Therefore, Potassium (K) has a larger atomic radius than Sodium (Na) due to the increased principal quantum number.
-
Period Comparison: Chlorine (Cl) and Argon (Ar) are in Period 3. As we move across a period, the atomic radius decreases due to the increasing effective nuclear charge. Therefore, Chlorine (Cl) has a larger atomic radius than Argon (Ar).
-
Group vs. Period: Now, we compare Potassium (K) and Chlorine (Cl). Potassium is in a lower period (Period 4) and possesses an additional electron shell compared to Chlorine (Period 3). The increased shielding and distance from the nucleus outweigh the slightly higher effective nuclear charge in Potassium. Consequently, Potassium (K) has a larger atomic radius than Chlorine (Cl).
Therefore, in this example, Potassium (K) has the largest atomic radius among Sodium (Na), Potassium (K), Chlorine (Cl), and Argon (Ar).
Advanced Considerations: Anomalies and Exceptions
While the general trends are predictable, some exceptions exist. These deviations are often attributed to specific electron configurations or inter-electronic repulsions. For instance, certain electron configurations might lead to more effective shielding or greater electron-electron repulsion than expected, slightly altering the atomic radius. These exceptions require a more in-depth analysis of electronic structure and are often beyond the scope of simple periodic trend comparisons.
Applications of Understanding Atomic Radius
Understanding atomic radius is crucial in various scientific fields:
-
Chemistry: Predicting the reactivity of elements, understanding bonding characteristics (ionic, covalent, metallic), and interpreting molecular geometries.
-
Materials Science: Designing materials with specific properties, such as strength, conductivity, and reactivity. The size of atoms influences the packing efficiency in solids, directly impacting material properties.
-
Physics: Modeling atomic interactions, understanding the behavior of matter in various states, and developing new technologies based on atomic-level manipulation.
-
Nuclear Physics: Understanding nuclear reactions and the stability of isotopes.
Conclusion: Predicting the Largest Atomic Radius
To determine which element possesses the largest atomic radius among a given set, follow these steps:
-
Identify the periods and groups: Locate the elements on the periodic table and note their periods and groups.
-
Apply periodic trends: Remember that atomic radius generally increases down a group and decreases across a period.
-
Compare elements: Systematically compare the elements, considering their positions on the periodic table and the impact of electron shells, effective nuclear charge, and electron-electron repulsion.
-
Consider anomalies: While periodic trends are reliable, be aware of potential exceptions due to variations in electronic configurations.
By understanding the underlying principles governing atomic size and applying the periodic trends, we can accurately predict which element will have the largest atomic radius in any given comparison. This knowledge is fundamental to understanding the behavior and properties of elements and their compounds. This detailed analysis highlights the importance of understanding not just the basic trends but also the subtle factors that contribute to the overall size of an atom. Further exploration into advanced concepts like electron configurations and quantum mechanics will enhance this understanding even further.
Latest Posts
Latest Posts
-
1 4 Pound Is How Many Ounces
Mar 28, 2025
-
Oxidation State Of Nitrogen In Ammonia
Mar 28, 2025
-
What Is A Subscript In A Chemical Equation
Mar 28, 2025
-
What Is The Charge On A Sulfide Ion
Mar 28, 2025
-
How To Multiply 2x2 Matrix By 2x1
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Elements Has The Largest Atomic Radius . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
