What Is The Charge On A Sulfide Ion
listenit
Mar 28, 2025 · 7 min read
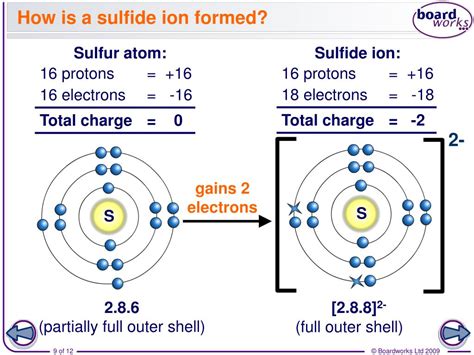
Table of Contents
What is the Charge on a Sulfide Ion? A Deep Dive into Sulfur's Anionic Behavior
Understanding the charge of a sulfide ion is fundamental to comprehending various chemical processes, from the formation of minerals to biological functions. This article delves deep into the electronic structure of sulfur, its tendency to form anions, and the specific charge carried by the sulfide ion. We'll explore the concepts behind ionic bonding, oxidation states, and the practical implications of sulfide's negative charge in different contexts.
The Electronic Structure of Sulfur: The Foundation for Anion Formation
Sulfur (S), element number 16 on the periodic table, resides in Group 16 (also known as the chalcogens or oxygen family). Its electronic configuration is [Ne] 3s²3p⁴. This means it has six valence electrons—electrons in the outermost shell that participate in chemical bonding. Atoms strive for a stable electron configuration, often resembling the nearest noble gas. For sulfur, achieving the stable octet configuration (eight valence electrons like argon) is energetically favorable.
To reach this stable state, sulfur readily gains two electrons. This electron gain transforms a neutral sulfur atom into a sulfide ion (S²⁻). This process is characteristic of non-metals, which tend to gain electrons to form anions (negatively charged ions). The strong electronegativity of sulfur contributes to its eagerness to accept electrons, making the formation of sulfide ions a highly prevalent phenomenon.
Electronegativity and the Drive for Electron Gain
Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. Sulfur's relatively high electronegativity (2.58 on the Pauling scale) indicates its strong tendency to pull electrons toward itself. When sulfur encounters an element with lower electronegativity (like many metals), it readily accepts electrons to form a stable octet, creating the negatively charged sulfide ion.
Ionic Bonding: The Role of the Sulfide Ion in Crystal Structures
The sulfide ion's negative charge plays a crucial role in ionic bonding. Ionic bonds are formed through the electrostatic attraction between oppositely charged ions. The strong attraction between the negatively charged sulfide ion (S²⁻) and positively charged metal cations (like Na⁺, Fe²⁺, or Pb²⁺) leads to the formation of ionic compounds known as sulfides.
Examples of Sulfide Minerals and Compounds
Many naturally occurring minerals are sulfides. These minerals are essential components of Earth's crust and play vital roles in various geological processes. Some examples include:
-
Pyrite (FeS₂): Also known as "fool's gold," pyrite is an iron sulfide with a distinctive cubic crystalline structure. The iron ions (Fe²⁺) are balanced by the negative charges from the disulfide (S₂²⁻) anion. Note that while the overall charge of the anion is -2, each sulfur atom within it technically has an oxidation state of -1.
-
Galena (PbS): This lead sulfide is an important ore for lead extraction. The strong ionic bond between Pb²⁺ and S²⁻ contributes to the mineral's stability.
-
Sphalerite (ZnS): A zinc sulfide mineral, sphalerite is a primary source of zinc. The 2+ charge of zinc cations neatly counterbalances the -2 charge of the sulfide ions.
-
Cinnabar (HgS): This mercury sulfide is a vibrant red mineral and a crucial mercury source. The mercury(II) cations and sulfide ions interact to form this intensely colored compound.
Crystal Lattice Structure and Sulfide Ion Placement
The sulfide ions are strategically positioned within the crystal lattice structures of these minerals. The arrangement maximizes electrostatic attraction while minimizing repulsion between similarly charged ions. Understanding the crystal structure is essential for predicting the physical and chemical properties of sulfide minerals.
Oxidation States: A Deeper Look at Sulfur's Charge
The concept of oxidation state helps in understanding the apparent charge on the sulfur atom in various chemical environments. While the sulfide ion (S²⁻) unequivocally carries a -2 charge, sulfur can exhibit different oxidation states depending on the bonding partners.
Variable Oxidation States of Sulfur
Sulfur is unique in that it can exhibit a wide range of oxidation states, from -2 (in sulfides) to +6 (in sulfuric acid, H₂SO₄). The oxidation state reflects the apparent charge an atom has in a molecule or ion, considering the electrons shared in covalent bonds as being assigned to the more electronegative atom.
-
-2 (Sulfide): This is the most common and stable negative oxidation state for sulfur, found in many metal sulfides.
-
0 (Elemental Sulfur): In its elemental form (S₈), sulfur's oxidation state is zero, as there is no net electron transfer.
-
+2, +4, +6: Higher oxidation states of sulfur are found in compounds like sulfur dioxide (SO₂, +4), sulfur trioxide (SO₃, +6), and sulfate ions (SO₄²⁻, +6). These higher oxidation states reflect sulfur's ability to form covalent bonds and share electrons with highly electronegative elements like oxygen.
Understanding the various oxidation states of sulfur helps in predicting the reactivity and chemical properties of different sulfur-containing compounds. It also provides valuable information in fields like geochemistry and environmental science, where sulfur transformations play a key role.
The Importance of Sulfide Ions in Biological Systems
Sulfide ions are not only prevalent in geological settings but also play significant roles in biological systems. While high concentrations of sulfide are toxic, it is a key component in various metabolic pathways.
Sulfide's Role in Metabolism
Sulfide is involved in sulfur metabolism, an essential process for various life forms. Certain bacteria utilize sulfide as an electron donor in anaerobic respiration, highlighting its significance in energy production within specific ecosystems.
The Toxicity of Sulfide
At elevated concentrations, hydrogen sulfide (H₂S), which arises from the ionization of sulfide ions in aqueous solutions, is highly toxic to many organisms. The toxicity stems from its ability to inhibit mitochondrial function, disrupting cellular respiration. This reinforces the crucial role of environmental regulation to maintain sulfide levels within safe limits.
Sulfide and its Impact on the Environment
Sulfide ions play a significant role in the global sulfur cycle, a biogeochemical cycle involving the movement of sulfur through different reservoirs (atmosphere, lithosphere, hydrosphere, biosphere). Human activities, such as the burning of fossil fuels and industrial processes, can significantly impact the sulfur cycle, often leading to increased atmospheric sulfur dioxide, which eventually leads to acid rain and other environmental issues.
Analytical Techniques for Detecting and Quantifying Sulfide Ions
Several analytical methods exist for detecting and quantifying sulfide ions in various samples. These techniques are crucial for monitoring sulfide levels in environmental samples, industrial processes, and biological studies.
Chemical Tests for Sulfide
Qualitative tests, such as lead acetate paper tests (which create a black precipitate of lead sulfide in the presence of sulfide), can provide a quick indication of the presence of sulfide. However, more precise quantitative measurements rely on instrumental techniques.
Instrumental Techniques for Sulfide Analysis
-
Ion chromatography: This technique separates ions based on their charge and size, allowing for the precise quantification of sulfide ions in a solution.
-
Spectrophotometry: Spectrophotometric methods utilize the absorbance of light by sulfide-containing complexes to determine the concentration of sulfide ions.
-
Electrochemical methods: Techniques like ion-selective electrodes (ISEs) are highly sensitive and can measure sulfide ion concentrations in various samples.
The choice of analytical technique depends on the specific requirements of the analysis, including the concentration range of sulfide ions, the nature of the sample matrix, and the desired level of precision and accuracy.
Conclusion: The Significance of a Simple Ion
The simple sulfide ion, with its seemingly straightforward -2 charge, plays a critical role in a vast array of chemical processes, both naturally occurring and human-engineered. From the formation of minerals and the structure of crystal lattices to its involvement in biological metabolism and its impact on the environment, understanding the properties and behavior of the sulfide ion is fundamental across multiple scientific disciplines. The information detailed in this article provides a comprehensive overview of the sulfide ion, highlighting its importance within the greater context of chemistry and beyond. Further research into specific areas like sulfide mineralogy, biogeochemistry, and environmental chemistry will reveal even more complexities and fascinating aspects of this ubiquitous anion.
Latest Posts
Latest Posts
-
Least Common Multiple 16 And 24
Mar 31, 2025
-
What Is The Name Of This Hydrocarbon
Mar 31, 2025
-
Columns Of Periodic Table Are Called
Mar 31, 2025
-
Difference Between Animal Mitosis And Plant Mitosis
Mar 31, 2025
-
What Is Half Of One And A Half Teaspoons
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is The Charge On A Sulfide Ion . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
