What Is A Subscript In A Chemical Equation
listenit
Mar 28, 2025 · 6 min read
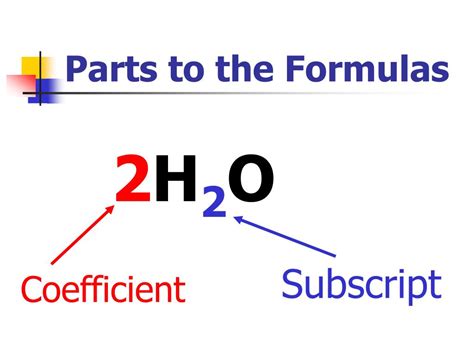
Table of Contents
- What Is A Subscript In A Chemical Equation
- Table of Contents
- What is a Subscript in a Chemical Equation? A Comprehensive Guide
- Understanding the Basics: Atoms, Molecules, and Subscripts
- The Significance of Subscripts in Chemical Equations
- Subscripts vs. Coefficients: A Clear Distinction
- Advanced Concepts: Polyatomic Ions and Subscripts
- Subscripts and Isotopes: A Subtle Distinction
- Practical Applications: Subscripts in Everyday Life
- Conclusion: Mastering Subscripts for Chemical Proficiency
- Latest Posts
- Latest Posts
- Related Post
What is a Subscript in a Chemical Equation? A Comprehensive Guide
Subscripts in chemical equations are not merely small numbers; they are fundamental components that dictate the composition and behavior of chemical substances. Understanding their role is crucial for comprehending chemical reactions and stoichiometry. This comprehensive guide delves into the intricacies of subscripts, clarifying their meaning, function, and significance in various chemical contexts.
Understanding the Basics: Atoms, Molecules, and Subscripts
Before diving into the complexities of subscripts, let's establish a foundational understanding of atoms and molecules. An atom is the smallest unit of an element that retains the chemical properties of that element. Elements are substances consisting of only one type of atom, such as oxygen (O), hydrogen (H), or carbon (C).
A molecule is a group of two or more atoms chemically bonded together. These atoms can be of the same element (like O₂ for oxygen gas) or different elements (like H₂O for water). The subscript in a chemical formula tells us how many atoms of a particular element are present in a single molecule.
For example:
- H₂O: This formula indicates one molecule of water, composed of two hydrogen atoms (indicated by the subscript 2 after H) and one oxygen atom (implied subscript of 1 after O).
- CO₂: This formula represents one molecule of carbon dioxide, consisting of one carbon atom (implied subscript of 1 after C) and two oxygen atoms (indicated by the subscript 2 after O).
- C₆H₁₂O₆: This formula represents one molecule of glucose, a sugar, composed of six carbon atoms, twelve hydrogen atoms, and six oxygen atoms.
The Significance of Subscripts in Chemical Equations
Subscripts are not just about counting atoms; they are critical for several reasons:
-
Determining the Molecular Formula: The subscript dictates the precise composition of a molecule. Altering a subscript changes the identity of the substance entirely. For example, CO (carbon monoxide) is a toxic gas, while CO₂ (carbon dioxide) is a gas vital for plant life. A simple change in subscript dramatically alters the chemical properties.
-
Balancing Chemical Equations: Subscripts play a vital role in balancing chemical equations. The Law of Conservation of Mass states that matter cannot be created or destroyed in a chemical reaction; only rearranged. Therefore, the number of atoms of each element must be the same on both sides (reactants and products) of a balanced chemical equation. Subscripts inform us about the initial number of atoms, guiding us in balancing the equation using coefficients (the numbers placed in front of molecules).
-
Stoichiometric Calculations: Subscripts are fundamental in stoichiometric calculations. Stoichiometry is the quantitative study of reactants and products in chemical reactions. Knowing the subscripts allows us to calculate the molar masses of compounds, determine the limiting reactant, and calculate theoretical yields of products.
-
Understanding Chemical Properties: The arrangement and number of atoms (as dictated by subscripts) directly influences the chemical and physical properties of a substance. For example, the difference in bonding between carbon atoms in diamond (strong covalent network) and graphite (layered structure) results from variations in their molecular structures, indirectly influenced by the implied subscripts in their simplest formulas.
Subscripts vs. Coefficients: A Clear Distinction
It's crucial to differentiate between subscripts and coefficients in chemical equations. As discussed earlier, subscripts indicate the number of atoms of a particular element within a single molecule. Coefficients, on the other hand, represent the number of molecules of a given substance involved in a reaction.
Example:
Consider the balanced equation for the combustion of methane:
CH₄ + 2O₂ → CO₂ + 2H₂O
-
Subscripts: The subscript '4' in CH₄ indicates four hydrogen atoms in one methane molecule. The subscript '2' in O₂ signifies two oxygen atoms in one oxygen molecule. Similarly, the subscripts in CO₂ and H₂O indicate the composition of those molecules.
-
Coefficients: The coefficient '1' (implied) before CH₄ means one molecule of methane is reacting. The coefficient '2' before O₂ means two molecules of oxygen are reacting. The coefficients '1' (implied) before CO₂ and '2' before H₂O indicate the number of molecules of carbon dioxide and water produced, respectively.
Advanced Concepts: Polyatomic Ions and Subscripts
The concept of subscripts extends to polyatomic ions. Polyatomic ions are groups of atoms covalently bonded together that carry a net electric charge. Examples include sulfate (SO₄²⁻), nitrate (NO₃⁻), and ammonium (NH₄⁺).
Subscripts within the polyatomic ion formula indicate the number of each atom within that ion. When a polyatomic ion appears multiple times in a chemical formula, parentheses are used to enclose the ion, and a subscript outside the parenthesis indicates the number of polyatomic ions present.
Example:
(NH₄)₂SO₄ (Ammonium sulfate)
Here, the subscript '2' outside the parenthesis indicates two ammonium ions (NH₄⁺). Each ammonium ion contains one nitrogen atom and four hydrogen atoms, as indicated by the subscripts within the parentheses. The formula also contains one sulfate ion (SO₄²⁻).
Subscripts and Isotopes: A Subtle Distinction
While subscripts predominantly deal with the number of atoms, it's important to note that they don't directly reflect the isotopic composition of the element. Isotopes are atoms of the same element with the same number of protons but different numbers of neutrons. For instance, carbon-12 (¹²C) and carbon-14 (¹⁴C) are isotopes of carbon.
The subscripts in chemical formulas represent the total number of atoms of each element, regardless of their isotopic variations. Isotopic information is generally not included in standard chemical formulas unless specifically relevant to a particular nuclear or isotopic analysis.
Practical Applications: Subscripts in Everyday Life
The significance of subscripts extends far beyond the classroom. They are fundamental to numerous real-world applications:
-
Pharmaceuticals: Accurate chemical formulas, relying heavily on subscripts, are critical in pharmaceutical development and manufacturing. Even slight changes in the number of atoms can drastically alter a drug's efficacy or toxicity.
-
Materials Science: Subscripts are essential in designing and characterizing materials with specific properties. The composition and stoichiometry, dictated by subscripts, determine the material's strength, conductivity, and other crucial features.
-
Environmental Science: Understanding chemical equations and the role of subscripts is vital for monitoring pollution levels, predicting the fate of pollutants in the environment, and developing remediation strategies.
-
Food Science: The chemical composition of food, governed by subscripts in the molecular formulas of its constituents, determines its nutritional value, taste, and shelf life.
Conclusion: Mastering Subscripts for Chemical Proficiency
Subscripts in chemical equations are not simply small numbers; they represent the fundamental building blocks of chemical compounds and are essential for understanding chemical reactions. A thorough grasp of their significance allows for accurate prediction of chemical behavior, facilitates stoichiometric calculations, and unlocks a deeper appreciation for the world around us at a molecular level. From balancing equations to understanding complex chemical interactions, mastery of subscripts is paramount for anyone pursuing a deeper understanding of chemistry. This understanding extends far beyond theoretical concepts, finding crucial applications in diverse scientific and technological fields.
Latest Posts
Latest Posts
-
How Many Protons Are In Carbon 14
Mar 31, 2025
-
How Are Pressure And Temp Related
Mar 31, 2025
-
What Is 2 4 In A Fraction
Mar 31, 2025
-
Organisms That Cannot Make Their Own Food
Mar 31, 2025
-
How Many Electrons Are In The 4th Energy Level
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is A Subscript In A Chemical Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
