How Many Electrons Are In The 4th Energy Level
listenit
Mar 31, 2025 · 5 min read
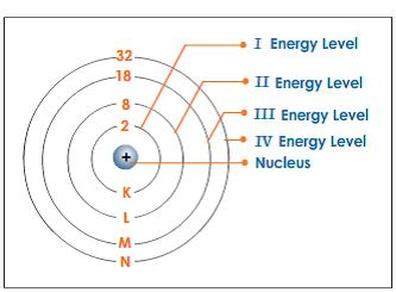
Table of Contents
How Many Electrons Are in the 4th Energy Level? A Deep Dive into Electron Configuration
The question of how many electrons can occupy the fourth energy level of an atom is a fundamental concept in chemistry and physics. Understanding this requires delving into the intricacies of electron configuration and the quantum mechanical model of the atom. While a simple answer exists, a complete understanding requires exploring the underlying principles. This article will provide not just the answer but also a comprehensive explanation, covering key concepts and related topics to solidify your grasp of atomic structure.
Understanding Energy Levels and Sublevels
Before we tackle the fourth energy level specifically, let's establish a foundation. Electrons in an atom don't simply orbit the nucleus randomly. They exist in specific energy levels, often depicted as shells surrounding the nucleus. These energy levels are quantized, meaning electrons can only occupy specific energy states and not exist in between. The further an energy level is from the nucleus, the higher its energy.
Each energy level, however, isn't just a single shell. It's further subdivided into sublevels or subshells, which are designated by the letters s, p, d, and f. These sublevels represent different shapes and orientations of electron orbitals. The number of sublevels within an energy level increases as the energy level increases.
- s sublevel: This sublevel has only one orbital, capable of holding a maximum of 2 electrons.
- p sublevel: This sublevel has three orbitals, each capable of holding 2 electrons, for a total of 6 electrons.
- d sublevel: This sublevel has five orbitals, holding a maximum of 10 electrons.
- f sublevel: This sublevel has seven orbitals, accommodating a maximum of 14 electrons.
The Fourth Energy Level: A Detailed Breakdown
Now, let's focus on the fourth energy level (n=4). This energy level contains all four sublevels: s, p, d, and f. To determine the total number of electrons it can hold, we simply add up the maximum number of electrons each sublevel can accommodate:
- 4s sublevel: Holds a maximum of 2 electrons.
- 4p sublevel: Holds a maximum of 6 electrons.
- 4d sublevel: Holds a maximum of 10 electrons.
- 4f sublevel: Holds a maximum of 14 electrons.
Therefore, the total number of electrons the fourth energy level can hold is 2 + 6 + 10 + 14 = 32 electrons.
Electron Configuration and the Aufbau Principle
The way electrons fill energy levels and sublevels is governed by the Aufbau principle, which states that electrons first occupy the lowest energy levels available. This principle, combined with Hund's rule (electrons fill orbitals individually before pairing up) and the Pauli exclusion principle (no two electrons can have the same four quantum numbers), dictates the electron configuration of an atom.
For example, the electron configuration of a neutral krypton atom (atomic number 36) shows the complete filling of the first three energy levels and a partial filling of the fourth: 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶. Note that even though the 4s sublevel fills before the 3d, the 4th energy level isn't completely filled until after the 3d is also complete.
Atoms beyond Krypton continue to fill the 4th energy level, with the 4f orbitals being filled last, in the Lanthanide series. The elements in this series showcase the full capacity of the 4th energy level to hold electrons.
Exceptions to the Rules: Orbital Stability
While the Aufbau principle provides a good general guideline, there are exceptions. In some cases, the energy levels are close enough that electron configurations may deviate slightly from the predicted order to achieve greater stability. This is often seen in transition metals and other elements with partially filled d or f orbitals. The slight energy differences between orbitals sometimes lead to anomalous electron configurations.
These exceptions are not random; they are a result of the intricate interplay of electron-electron repulsions and the shielding effect of inner electrons. The specific energy of an orbital depends on the atom and the nuclear charge it encounters.
Quantum Numbers and Orbital Shapes
To further refine our understanding, we can delve into quantum numbers, which describe the properties of electrons within an atom:
- Principal Quantum Number (n): This number designates the energy level (n=1, 2, 3, 4...). For the fourth energy level, n=4.
- Azimuthal Quantum Number (l): This number indicates the sublevel (l=0 for s, l=1 for p, l=2 for d, l=3 for f).
- Magnetic Quantum Number (ml): This specifies the orbital within a sublevel.
- Spin Quantum Number (ms): This describes the intrinsic angular momentum of the electron, which can be +1/2 or -1/2 (spin up or spin down).
Each electron within an atom is uniquely identified by its set of four quantum numbers. These numbers determine the electron's energy, shape of its orbital, and its orientation in space.
Implications in Chemical Bonding and Reactivity
The number of electrons in the outermost energy level (valence electrons) directly impacts an atom's chemical behavior. Elements with similar valence electron configurations tend to exhibit similar chemical properties. For elements with electrons in the fourth energy level, this impacts their reactivity and bonding characteristics. For example, the transition metals with incompletely filled 3d and 4s orbitals exhibit a diverse range of oxidation states and form complex ions.
Conclusion: Beyond the Simple Answer
While the simple answer is that the fourth energy level can hold a maximum of 32 electrons, understanding the underlying principles of electron configuration, quantum mechanics, and the Aufbau principle is crucial for a truly comprehensive grasp of atomic structure. The distribution of electrons within the energy levels profoundly influences an atom's chemical properties and its interactions with other atoms. This detailed exploration goes beyond simply stating the number; it provides a deeper appreciation of the complex and fascinating world of atomic structure and quantum mechanics. Further exploration into the specifics of orbital shapes, energies, and the exceptions to the rules will reveal a richer and more complete understanding of the fundamental building blocks of matter. This deeper understanding is essential for advancements in many fields, including materials science, medicine, and nanotechnology.
Latest Posts
Latest Posts
-
What Happens To Pressure When Volume Increases
Apr 02, 2025
-
What Is Lcm Of 10 And 12
Apr 02, 2025
-
25 Of What Number Is 6
Apr 02, 2025
-
What Is The Circumference Of A Circle With Radius 3
Apr 02, 2025
-
36 Is What Percent Of 45
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Are In The 4th Energy Level . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
