What Happens To Pressure When Volume Increases
listenit
Apr 02, 2025 · 6 min read
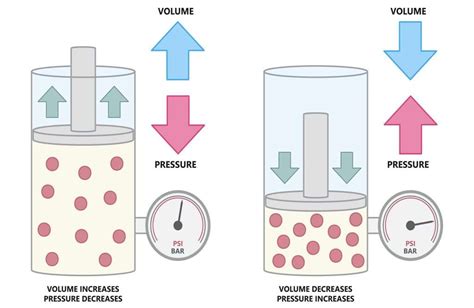
Table of Contents
- What Happens To Pressure When Volume Increases
- Table of Contents
- What Happens to Pressure When Volume Increases? An In-Depth Exploration of Boyle's Law
- Boyle's Law: The Inverse Relationship Between Pressure and Volume
- Understanding the Microscopic Perspective
- Factors Affecting the Pressure-Volume Relationship
- Temperature: A Crucial Variable
- The Nature of the Gas: Ideal vs. Real Gases
- Amount of Gas: Keeping it Constant
- Practical Applications of Boyle's Law
- Respiratory System: Breathing Mechanics
- Diving and Underwater Activities: Pressure Changes with Depth
- Internal Combustion Engines: Powering Vehicles
- Medical Applications: Ventilation and Anesthesia
- Meteorology: Weather Patterns and Atmospheric Pressure
- Beyond Boyle's Law: More Complex Gas Laws
- Charles's Law: Temperature and Volume
- Gay-Lussac's Law: Temperature and Pressure
- The Ideal Gas Law: Combining the Laws
- Conclusion: A Cornerstone of Physics and Beyond
- Latest Posts
- Latest Posts
- Related Post
What Happens to Pressure When Volume Increases? An In-Depth Exploration of Boyle's Law
Understanding the relationship between pressure and volume is fundamental to comprehending various physical phenomena, from the mechanics of breathing to the operation of internal combustion engines. This article delves into the inverse relationship between pressure and volume, primarily focusing on Boyle's Law, and explores its implications across various scientific disciplines. We'll examine the underlying principles, practical applications, and limitations of this crucial gas law.
Boyle's Law: The Inverse Relationship Between Pressure and Volume
Boyle's Law, also known as Boyle-Mariotte Law, states that the absolute pressure exerted by a given mass of an ideal gas is inversely proportional to the volume it occupies if the temperature and amount of gas remain unchanged within a closed system. In simpler terms: as volume increases, pressure decreases, and vice versa. This relationship is represented mathematically as:
P₁V₁ = P₂V₂
Where:
- P₁ represents the initial pressure
- V₁ represents the initial volume
- P₂ represents the final pressure
- V₂ represents the final volume
This equation highlights the inverse proportionality – if you double the volume (V₂ = 2V₁), the pressure will be halved (P₂ = P₁/2). Conversely, halving the volume will double the pressure.
Understanding the Microscopic Perspective
The inverse relationship between pressure and volume can be explained at a microscopic level by considering the behavior of gas molecules. Pressure is essentially the result of countless gas molecules colliding with the walls of their container. When the volume increases, the molecules have more space to move around. This increased space leads to fewer collisions per unit time with the container walls, resulting in a decrease in pressure. Conversely, reducing the volume confines the molecules to a smaller space, increasing the frequency of collisions and thus raising the pressure.
Factors Affecting the Pressure-Volume Relationship
While Boyle's Law provides a fundamental understanding, it's crucial to acknowledge its limitations and the factors that can influence the pressure-volume relationship beyond the ideal gas scenario.
Temperature: A Crucial Variable
Boyle's Law holds true only when the temperature remains constant. Changes in temperature significantly impact the kinetic energy of gas molecules. Increased temperature leads to faster-moving molecules, resulting in more frequent and forceful collisions with the container walls, thus increasing pressure even if the volume stays the same. Conversely, lower temperatures lead to slower molecules and reduced pressure. This underscores the importance of isothermal conditions (constant temperature) for Boyle's Law to accurately predict the pressure-volume relationship.
The Nature of the Gas: Ideal vs. Real Gases
Boyle's Law is based on the ideal gas model, which assumes that gas molecules have negligible volume and exert no intermolecular forces. However, real gases deviate from this ideal behavior, especially at high pressures and low temperatures. At high pressures, the volume occupied by the gas molecules themselves becomes significant compared to the total volume of the container, leading to a deviation from the inverse proportionality predicted by Boyle's Law. Similarly, intermolecular forces, which are attractive at close range, become more prominent at low temperatures and high pressures, further affecting the accuracy of Boyle's Law's predictions.
Amount of Gas: Keeping it Constant
The law assumes a constant amount of gas within the closed system. Adding or removing gas molecules changes the number of collisions with the container walls, directly affecting the pressure irrespective of the volume. This highlights the importance of maintaining a closed system with a fixed number of gas particles for Boyle's Law to be applicable.
Practical Applications of Boyle's Law
Boyle's Law has far-reaching applications across numerous fields.
Respiratory System: Breathing Mechanics
Our lungs exemplify Boyle's Law in action. Inhalation involves expanding the chest cavity, increasing the lung volume. This increase in volume leads to a decrease in pressure inside the lungs, creating a pressure difference that draws air into the lungs. Exhalation reverses this process; the chest cavity contracts, decreasing lung volume and increasing pressure, forcing air out of the lungs.
Diving and Underwater Activities: Pressure Changes with Depth
As divers descend deeper underwater, the pressure of the surrounding water increases significantly. This increase in pressure compresses the air in their scuba tanks, reducing its volume. Divers need to understand this relationship to manage their air supply effectively and avoid decompression sickness.
Internal Combustion Engines: Powering Vehicles
The internal combustion engine relies heavily on the principles of Boyle's Law. The compression stroke reduces the volume of the fuel-air mixture in the cylinder, significantly increasing its pressure. This high-pressure mixture is then ignited, causing a rapid expansion that drives the piston and generates power.
Medical Applications: Ventilation and Anesthesia
Boyle's Law is critical in medical devices like ventilators. These machines control the pressure and volume of air delivered to a patient's lungs, ensuring adequate ventilation, especially for individuals with respiratory problems. In anesthesia, understanding how pressure and volume affect gas delivery is vital for administering anesthetic gases safely and effectively.
Meteorology: Weather Patterns and Atmospheric Pressure
Atmospheric pressure changes with altitude. At higher altitudes, the volume of air above a given point decreases, leading to a reduction in pressure. This pressure difference drives weather patterns and air movement in the atmosphere.
Beyond Boyle's Law: More Complex Gas Laws
While Boyle's Law provides a solid foundation, more complex gas laws are needed to accurately describe the behavior of gases under various conditions.
Charles's Law: Temperature and Volume
Charles's Law describes the relationship between volume and temperature when pressure remains constant. It states that the volume of a gas is directly proportional to its absolute temperature.
Gay-Lussac's Law: Temperature and Pressure
Gay-Lussac's Law shows the direct relationship between pressure and temperature when volume is kept constant. An increase in temperature leads to a corresponding increase in pressure.
The Ideal Gas Law: Combining the Laws
The Ideal Gas Law combines Boyle's Law, Charles's Law, and Avogadro's Law to provide a comprehensive equation describing the behavior of ideal gases:
PV = nRT
Where:
- P is pressure
- V is volume
- n is the amount of gas (in moles)
- R is the ideal gas constant
- T is temperature (in Kelvin)
The Ideal Gas Law is a more accurate representation of gas behavior than Boyle's Law alone, but it still relies on the assumption of ideal gas behavior.
Conclusion: A Cornerstone of Physics and Beyond
The relationship between pressure and volume, as primarily described by Boyle's Law, is a fundamental concept in physics and chemistry. Its applications span various scientific disciplines, from understanding the mechanics of breathing to designing high-performance engines and sophisticated medical devices. While the ideal gas model offers a simplified understanding, it's crucial to acknowledge its limitations and consider the influence of temperature, the nature of the gas, and the amount of gas present for a more complete and accurate description of the pressure-volume relationship in real-world scenarios. The exploration of Boyle's Law and its extensions provides a vital framework for understanding the behavior of gases and their significant role in numerous natural and technological processes. By grasping these fundamental principles, we gain a deeper understanding of the physical world around us.
Latest Posts
Latest Posts
-
Is Boron A Gas Solid Or Liquid
Apr 05, 2025
-
How Do You Factor 2x 2 7x 3
Apr 05, 2025
-
Which Group Of Metals Are The Most Reactive
Apr 05, 2025
-
How To Find The Perimeter Of A Right Angle Triangle
Apr 05, 2025
-
How Many Valence Electrons Are In Cl
Apr 05, 2025
Related Post
Thank you for visiting our website which covers about What Happens To Pressure When Volume Increases . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
