Which Element Has The Larger Ionization Energy
listenit
Mar 31, 2025 · 5 min read
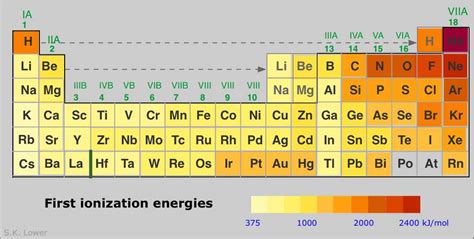
Table of Contents
Which Element Has the Larger Ionization Energy? A Deep Dive into Atomic Structure and Periodic Trends
Understanding ionization energy is crucial for comprehending the behavior of elements and their interactions. This article delves into the factors influencing ionization energy, exploring the periodic trends and providing examples to illustrate which elements generally possess higher ionization energies. We'll also explore exceptions to these trends and the nuances behind ionization energy's complexities.
What is Ionization Energy?
Ionization energy (IE), also known as ionization potential, is the minimum amount of energy required to remove the most loosely bound electron from a neutral gaseous atom or ion. This process results in the formation of a positively charged ion (cation). The first ionization energy (IE₁) refers to the removal of the first electron, the second ionization energy (IE₂) refers to the removal of the second electron, and so on. Each successive ionization energy is always greater than the previous one because removing an electron leaves behind a more positively charged ion, making it harder to remove subsequent electrons.
Key Characteristics of Ionization Energy:
- Endothermic Process: Ionization is always an endothermic process, meaning it requires energy input.
- Gaseous State: The ionization energy is measured for atoms in the gaseous state to avoid interatomic interactions that could affect the measurement.
- Increasing Values: Successive ionization energies (IE₂, IE₃, etc.) always increase.
- Units: Ionization energy is typically expressed in kilojoules per mole (kJ/mol) or electronvolts (eV).
Factors Affecting Ionization Energy
Several factors govern the magnitude of an element's ionization energy:
1. Nuclear Charge:
The stronger the positive charge of the nucleus, the more strongly it attracts the electrons, increasing the ionization energy. Elements with higher atomic numbers generally have higher nuclear charges and therefore higher ionization energies.
2. Atomic Radius:
The distance between the nucleus and the outermost electrons (atomic radius) plays a vital role. A smaller atomic radius means the outermost electrons are closer to the nucleus and experience a stronger attractive force, leading to a higher ionization energy. Elements with smaller atomic radii generally exhibit higher ionization energies.
3. Shielding Effect:
Inner electrons shield the outermost electrons from the full positive charge of the nucleus. This shielding effect reduces the effective nuclear charge experienced by the outermost electrons. Elements with more inner electrons experience a greater shielding effect, lowering their ionization energy. The number of inner electrons and their arrangement in electron shells influences the magnitude of the shielding effect.
4. Electron Configuration:
The arrangement of electrons in different subshells (s, p, d, f) affects ionization energy. Electrons in s subshells are generally held more tightly than those in p subshells, which are held more tightly than those in d subshells, and so on. A full or half-filled subshell exhibits extra stability, requiring more energy to remove an electron. This phenomenon is due to electron-electron repulsions and exchange energy.
Periodic Trends in Ionization Energy
Ionization energy follows clear periodic trends across the periodic table:
1. Across a Period (Left to Right):
Ionization energy generally increases as you move from left to right across a period. This is because the nuclear charge increases while the shielding effect remains relatively constant. The electrons are added to the same principal energy level, so the atomic radius decreases, leading to a stronger attraction between the nucleus and electrons.
2. Down a Group (Top to Bottom):
Ionization energy generally decreases as you move down a group. This is primarily due to the increasing atomic radius. As you go down a group, electrons are added to higher principal energy levels, farther from the nucleus. The increased shielding effect also contributes to the decrease in ionization energy.
Exceptions to the Trends
While the general trends are predictable, exceptions exist due to the complexities of electron configurations and electron-electron repulsions. For example:
- Group 13 Elements: The ionization energy of Group 13 (Boron group) elements is lower than expected compared to their preceding elements in the period. This is because the electron being removed is from a p subshell, which is slightly less tightly bound than the s subshell.
- Group 16 Elements: The ionization energy of oxygen is lower than nitrogen. This is attributed to the electron-electron repulsion in the p subshell of oxygen, where two electrons occupy the same orbital. The repulsion makes it easier to remove one electron from oxygen than from nitrogen, which has a half-filled p subshell.
Comparing Ionization Energies: Specific Examples
Let's compare the ionization energies of specific elements to illustrate the concepts discussed:
Helium (He) vs. Lithium (Li): Helium has a higher ionization energy than Lithium. Helium has a smaller atomic radius and a higher nuclear charge, resulting in a stronger attraction to its electrons.
Nitrogen (N) vs. Oxygen (O): Nitrogen has a slightly higher first ionization energy than oxygen. This is an exception to the general trend due to the half-filled p subshell in nitrogen, which provides extra stability.
Sodium (Na) vs. Chlorine (Cl): Chlorine has a significantly higher ionization energy than sodium. Chlorine is located further to the right and closer to the top of the periodic table, resulting in a smaller atomic radius, stronger nuclear charge, and less shielding.
Fluorine (F) vs. Neon (Ne): Neon has a higher ionization energy than Fluorine. Although both are in the same period, Neon's completely filled electron shell provides exceptional stability, requiring substantially more energy to remove an electron.
Applications of Ionization Energy
The understanding of ionization energy has numerous applications in various fields:
- Chemistry: Predicting reactivity, forming chemical bonds, understanding the properties of compounds.
- Physics: Analyzing spectral lines, studying atomic structure and electronic transitions.
- Materials Science: Designing materials with specific electronic properties.
- Analytical Chemistry: Identifying elements using techniques like mass spectrometry.
Conclusion
Ionization energy is a fundamental property that significantly influences the chemical and physical behavior of elements. While general trends exist across the periodic table, exceptions can arise due to the complexities of electronic configurations and electron-electron interactions. Understanding these factors and their interplay is crucial for comprehending the behavior of atoms and molecules, with far-reaching implications across various scientific disciplines. The interplay of nuclear charge, atomic radius, shielding, and electron configuration dictates which element ultimately boasts a larger ionization energy in any given comparison. Thorough analysis, considering these nuanced factors, is key to accurately predicting and understanding this essential atomic property.
Latest Posts
Latest Posts
-
How To Find The Secant Line
Apr 01, 2025
-
What Is 2 To The Fifth Power
Apr 01, 2025
-
How Many Electrons Can The First Shell Hold
Apr 01, 2025
-
15 6 As A Mixed Number
Apr 01, 2025
-
What Two Monosaccharides Make Up Sucrose
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Which Element Has The Larger Ionization Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
