What Two Monosaccharides Make Up Sucrose
listenit
Apr 01, 2025 · 6 min read
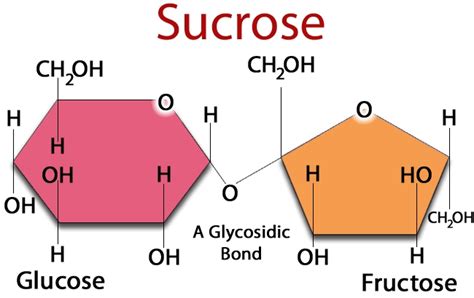
Table of Contents
What Two Monosaccharides Make Up Sucrose? A Deep Dive into the Chemistry and Biology of Table Sugar
Sucrose, the common table sugar we use daily, isn't a simple sugar itself. Instead, it's a disaccharide, meaning it's composed of two simpler sugars, or monosaccharides, bonded together. Understanding these monosaccharides and the bond that unites them is key to appreciating sucrose's properties and its role in biology and the food industry. This article will delve deep into the composition of sucrose, exploring its constituent monosaccharides, the chemical bonds involved, and its importance in various biological and culinary contexts.
The Building Blocks: Glucose and Fructose
Sucrose is formed from the combination of two monosaccharides: glucose and fructose. These are both hexoses, meaning they each contain six carbon atoms, and are isomers, sharing the same chemical formula (C<sub>6</sub>H<sub>12</sub>O<sub>6</sub>) but possessing different structural arrangements. This difference in structure leads to distinct chemical properties and functions in biological systems.
Glucose: The Universal Fuel
Glucose is arguably the most important monosaccharide in biology. It serves as the primary energy source for most living organisms. Plants produce glucose through photosynthesis, and animals obtain glucose through the digestion of carbohydrates. Glucose is readily absorbed into the bloodstream and utilized by cells to generate ATP (adenosine triphosphate), the main energy currency of the cell.
Key characteristics of glucose include:
-
Structure: Glucose exists in both linear and cyclic forms, with the cyclic form predominantly found in biological systems. This cyclic structure can exist as two anomers, α-glucose and β-glucose, differing in the orientation of the hydroxyl group on the first carbon atom. This seemingly small difference has significant consequences for the properties of polysaccharides formed from glucose units.
-
Metabolic Importance: Glucose metabolism is a central pathway in cellular respiration, providing energy for a wide range of cellular processes. The breakdown of glucose via glycolysis, the citric acid cycle, and oxidative phosphorylation yields large amounts of ATP.
-
Storage Forms: Excess glucose is stored in the body as glycogen in animals and starch in plants. These storage forms allow organisms to maintain a constant supply of glucose even when dietary intake is insufficient.
Fructose: The Fruit Sugar
Fructose, also known as fruit sugar or levulose, is another common hexose found in fruits and honey. It's sweeter than glucose, contributing to the sweetness of many fruits and processed foods. While fructose can be metabolized by the body, its metabolism differs from that of glucose, primarily occurring in the liver.
Key characteristics of fructose include:
-
Structure: Like glucose, fructose can exist in both linear and cyclic forms, but its cyclic form is a five-membered ring (furanose) rather than the six-membered ring (pyranose) found in glucose. This structural difference significantly impacts its reactivity and its interaction with enzymes.
-
Sweetness: Fructose is significantly sweeter than glucose, making it a desirable sweetener in the food industry. However, excessive consumption of fructose has been linked to various health problems, including metabolic syndrome and liver disease.
-
Metabolic Pathway: Unlike glucose, which is utilized by most cells, fructose is primarily metabolized in the liver. This can lead to different metabolic consequences compared to glucose metabolism, particularly with high fructose consumption.
The Glycosidic Bond: Uniting Glucose and Fructose
The glucose and fructose molecules in sucrose are linked together by a glycosidic bond. This is a covalent bond formed between the hemiacetal group of one monosaccharide and the hydroxyl group of another monosaccharide. Specifically, in sucrose, the bond is formed between the C1 carbon of α-glucose and the C2 carbon of β-fructose. This specific linkage creates a unique disaccharide with specific properties.
Understanding the glycosidic bond is crucial because:
-
It determines the properties of sucrose: The type of glycosidic bond (α-1,2-glycosidic bond in this case) influences the digestibility, sweetness, and other chemical properties of sucrose.
-
It impacts enzymatic breakdown: Specific enzymes are required to break the glycosidic bond and release the constituent monosaccharides. The α-1,2-glycosidic linkage in sucrose is readily hydrolyzed by the enzyme sucrase, found in the intestinal brush border, allowing for the absorption of glucose and fructose.
-
It influences the formation of other carbohydrates: Similar glycosidic bonds are found in other disaccharides (like lactose and maltose) and polysaccharides (like starch and cellulose), connecting monosaccharide units to form larger carbohydrate structures.
The Importance of Sucrose
Sucrose plays several significant roles in biology and industry:
Biological Roles:
-
Plant Energy Storage: Sucrose acts as a major transport form of sugar in plants, moving from photosynthetic leaves to other parts of the plant. It's also a storage carbohydrate in some plants.
-
Energy Source: Upon digestion, sucrose is broken down into glucose and fructose, providing a readily available energy source for animals and humans.
Industrial Roles:
-
Sweetener: Sucrose is a widely used sweetener in food and beverage industries, contributing to the sweetness and palatability of various products.
-
Preservative: High concentrations of sucrose can act as a preservative, inhibiting microbial growth and extending the shelf life of food products.
-
Raw Material: Sucrose serves as a raw material in the production of various other products, including ethanol, citric acid, and other chemicals.
Health Implications of Sucrose Consumption
While sucrose provides energy, excessive consumption is associated with several health problems:
-
Weight Gain: Sucrose is calorie-dense, contributing to weight gain if consumed in excess.
-
Dental Cavities: Sucrose provides a substrate for bacterial fermentation in the mouth, leading to dental caries (cavities).
-
Metabolic Disorders: High sucrose intake has been linked to an increased risk of metabolic syndrome, type 2 diabetes, and cardiovascular diseases. This is partly attributed to the fructose component and its unique metabolic pathway in the liver.
-
Nutrient Deficiency: High sucrose intake can displace the consumption of nutrient-rich foods, leading to nutrient deficiencies.
Therefore, moderation is key when it comes to sucrose consumption. A balanced diet that includes a variety of nutrient-rich foods and limits added sugars is crucial for maintaining overall health.
Conclusion: Beyond the Sweetness
Sucrose, though seemingly simple as table sugar, reveals a fascinating complexity in its chemical structure and biological roles. Its composition of glucose and fructose, united by a specific glycosidic bond, determines its properties and functions. Understanding the chemistry of sucrose and its impact on human health is essential for making informed dietary choices and appreciating the importance of carbohydrate metabolism in living organisms. While sucrose offers readily available energy, mindful consumption is crucial for preventing health risks associated with excessive sugar intake. Future research continues to explore the intricacies of sucrose metabolism and its long-term effects on human health. The ongoing investigation into the impacts of sugar consumption and the development of alternative sweeteners highlights the continuous importance of understanding this seemingly simple molecule.
Latest Posts
Latest Posts
-
How Many Quarts Are In 12 Cups
Apr 02, 2025
-
Stores The Genetic Information Of The Cell
Apr 02, 2025
-
The Quotient Of 14 And 7
Apr 02, 2025
-
What Happens To An Atom During A Chemical Reaction
Apr 02, 2025
-
How To Find N In Pv Nrt
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Two Monosaccharides Make Up Sucrose . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
