How Many Electrons Can The First Shell Hold
listenit
Apr 01, 2025 · 6 min read
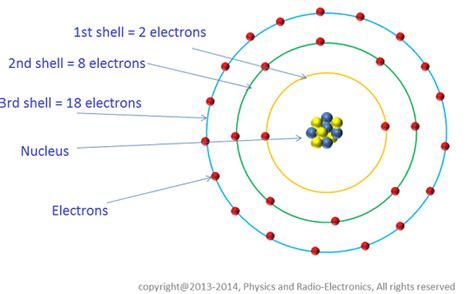
Table of Contents
How Many Electrons Can the First Shell Hold? A Deep Dive into Atomic Structure
Understanding the structure of an atom is fundamental to grasping the principles of chemistry and physics. A key aspect of this understanding involves knowing how electrons are arranged within an atom. This article delves deep into the question: how many electrons can the first shell hold? We'll explore the underlying principles of electron configuration, quantum numbers, and the implications of this limit on the properties of elements.
The Basics: Shells, Subshells, and Orbitals
Before answering the central question, let's establish some foundational concepts. Atoms consist of a nucleus containing protons and neutrons, surrounded by a cloud of electrons. These electrons aren't randomly distributed; they occupy specific energy levels, often visualized as shells or energy levels. These shells are further divided into subshells, denoted by the letters s, p, d, and f. Each subshell contains one or more orbitals, which are regions of space where there's a high probability of finding an electron.
- Shells (n): Represented by the principal quantum number (n), which can be any positive integer (1, 2, 3...). The shell number indicates the energy level; higher n values mean higher energy.
- Subshells (l): For a given shell (n), the number of subshells is equal to n. These are designated by the azimuthal quantum number (l), ranging from 0 to n-1. l = 0 corresponds to the s subshell, l = 1 to the p subshell, l = 2 to the d subshell, and l = 3 to the f subshell.
- Orbitals (ml): Each subshell contains a specific number of orbitals, determined by the magnetic quantum number (ml). The values of ml range from -l to +l, including 0. This means an s subshell has 1 orbital, a p subshell has 3 orbitals, a d subshell has 5 orbitals, and an f subshell has 7 orbitals.
- Electron Spin (ms): Each orbital can hold a maximum of two electrons, provided they have opposite spins. This is described by the spin quantum number (ms), which can be +1/2 or -1/2. This is known as the Pauli Exclusion Principle.
The First Shell: n=1
The first shell (n=1) is the closest to the nucleus and has the lowest energy. According to the rules outlined above:
- Number of subshells: Since n=1, there's only one subshell (l = 0, the s subshell).
- Number of orbitals: The s subshell contains only one orbital (ml = 0).
- Maximum number of electrons: Because each orbital can hold a maximum of two electrons (due to opposite spins), the first shell can hold a maximum of two electrons.
The Significance of the First Shell's Electron Capacity
The fact that the first shell can only hold two electrons has profound implications for the properties of elements and the periodic table. Hydrogen (H) and Helium (He) are the only elements whose electrons completely fill the first shell. Hydrogen has one electron in its first shell, while Helium has two. This complete first shell contributes to the extraordinary stability and inertness of Helium, a noble gas.
Electron Configuration and the Aufbau Principle
The arrangement of electrons in an atom is known as its electron configuration. The Aufbau principle guides this arrangement, stating that electrons fill the lowest energy levels first. This means that before electrons can occupy higher shells, the lower shells must be filled. This explains why hydrogen and helium have only electrons in the first shell.
Beyond the First Shell: Building Up the Periodic Table
As we move beyond helium, the second shell (n=2) begins to fill. The second shell has two subshells: the 2s subshell (one orbital, holding two electrons) and the 2p subshell (three orbitals, holding six electrons). This pattern continues with higher shells, each having more subshells and orbitals, leading to the increasing complexity and diversity of elements in the periodic table.
The Second Shell (n=2):
The second shell can hold a maximum of 8 electrons (2 in the 2s subshell and 6 in the 2p subshell). This capacity directly influences the chemical behavior of elements in the second row of the periodic table, including Lithium, Beryllium, Boron, Carbon, Nitrogen, Oxygen, Fluorine, and Neon. Neon, with a filled second shell, is also a noble gas, highlighting the stability associated with filled electron shells.
Higher Shells (n=3, 4, etc.):
The third shell (n=3) can accommodate up to 18 electrons, and the pattern continues, with even higher capacities for subsequent shells. The increasing number of electrons and the complexity of orbital arrangements contribute to the rich diversity of chemical properties observed across the periodic table.
Quantum Mechanics and the Explanation for Electron Shell Capacity
The precise number of electrons that each shell can hold is a direct consequence of the quantum mechanical model of the atom. The quantum numbers (n, l, ml, ms) arise from the solution to the Schrödinger equation, which describes the behavior of electrons in atoms. The limitations on the quantum numbers directly translate into limitations on the number of orbitals and, consequently, the maximum number of electrons a shell can accommodate.
The specific mathematical solutions dictate the shapes and energies of the orbitals, and the Pauli Exclusion Principle, a fundamental postulate of quantum mechanics, states that no two electrons in an atom can have the same set of four quantum numbers. This is what restricts the number of electrons within a given shell and subshell.
Applications and Importance
Understanding the electron shell capacity is crucial in various fields:
- Chemistry: Predicting chemical bonding, reactivity, and the formation of molecules relies heavily on the understanding of electron configurations and the tendency of atoms to achieve stable electron arrangements, often by filling shells.
- Physics: The principles of atomic structure are foundational to nuclear physics, material science, and condensed matter physics.
- Technology: The behavior of electrons in various materials is directly related to the electronic properties of these materials, which are vital in the development and function of modern electronics, semiconductors, and lasers.
Conclusion: The Two-Electron Limit of the First Shell
In summary, the first electron shell can hold a maximum of two electrons. This seemingly simple fact is a fundamental concept in chemistry and physics, stemming from the quantum mechanical nature of the atom. The capacity of electron shells directly influences the properties of elements, their reactivity, and the formation of chemical bonds. Understanding the electron shell structure allows us to interpret the periodic table and predict the behavior of atoms and molecules. This foundational knowledge is essential for numerous scientific disciplines and technological advancements. The stability conferred by a filled first shell, exemplified by Helium's inertness, exemplifies the profound impact of this seemingly small number on the vast landscape of chemistry and the universe itself.
Latest Posts
Latest Posts
-
What Is The Square Root Of 105
Apr 02, 2025
-
What Two Characteristics Of Living Things Do Viruses Exhibit
Apr 02, 2025
-
How Many Quarts Are In 12 Cups
Apr 02, 2025
-
Stores The Genetic Information Of The Cell
Apr 02, 2025
-
The Quotient Of 14 And 7
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Can The First Shell Hold . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
