Which Characteristic Is Given By The Angular Momentum Quantum Number
listenit
Mar 18, 2025 · 6 min read
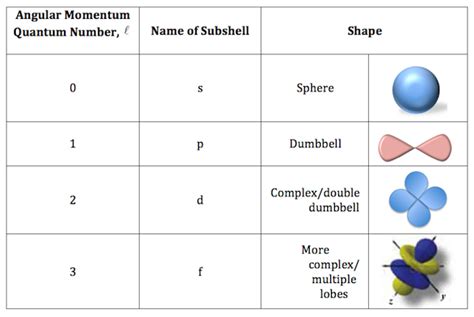
Table of Contents
Which Characteristic is Given by the Angular Momentum Quantum Number?
The angular momentum quantum number, denoted as l, is a crucial component in describing the behavior of electrons within an atom. It doesn't directly tell us the magnitude of the angular momentum (that requires a more complex calculation involving both l and Planck's constant), but it does define a fundamental characteristic: the shape of an electron's orbital. Understanding this characteristic is vital to comprehending atomic structure, chemical bonding, and the periodic properties of elements. This article will delve deep into the role of the angular momentum quantum number, exploring its relationship to orbital shapes, energy levels, and its contribution to the overall quantum mechanical description of the atom.
Understanding the Quantum Numbers
Before we dive into the specifics of the angular momentum quantum number (l), let's briefly review the other quantum numbers and their roles:
-
Principal Quantum Number (n): This number describes the electron's energy level and its average distance from the nucleus. Higher values of n indicate higher energy levels and greater distances. n can be any positive integer (1, 2, 3, ...).
-
Azimuthal Quantum Number (l): This is our focus, the angular momentum quantum number. It determines the shape of the electron's orbital and is related to the orbital angular momentum. It can take integer values from 0 to n - 1.
-
Magnetic Quantum Number (ml): This specifies the orientation of the orbital in space. It can take integer values from -l to +l, including 0.
-
Spin Quantum Number (ms): This describes the intrinsic angular momentum of the electron, often visualized as the electron "spinning" on its axis. It can only have two values: +1/2 or -1/2.
The Angular Momentum Quantum Number (l) and Orbital Shapes
The angular momentum quantum number (l) directly dictates the shape of the electron's atomic orbital. Each value of l corresponds to a specific orbital shape:
-
l = 0: s orbital (spherical): The s orbital is spherically symmetric; its electron density is distributed uniformly in all directions around the nucleus. This means the probability of finding the electron at a given distance from the nucleus is independent of direction. The 1s orbital is the smallest and closest to the nucleus, while higher n values (like 2s, 3s) represent larger, higher-energy s orbitals with additional radial nodes.
-
l = 1: p orbital (dumbbell-shaped): p orbitals are characterized by their dumbbell shape, with two lobes of electron density on opposite sides of the nucleus, separated by a node (a region of zero electron density) at the nucleus. For a given principal quantum number n, there are three p orbitals, designated as px, py, and pz, oriented along the x, y, and z axes respectively.
-
l = 2: d orbital (more complex shapes): d orbitals have more complex shapes than s and p orbitals. There are five d orbitals for each n level (with n ≥ 3), exhibiting a variety of shapes including cloverleaf and donut-like configurations. These different orientations are crucial in understanding the directional nature of chemical bonds in transition metal complexes.
-
l = 3: f orbital (even more complex shapes): f orbitals are even more intricate in shape, with seven orbitals per n level (with n ≥ 4). Their complex shapes are important in explaining the properties of lanthanides and actinides.
Connecting l to Orbital Angular Momentum
While l doesn't directly give the magnitude of the angular momentum, it's closely related. The magnitude of the orbital angular momentum is given by:
L = √[l(l+1)]ħ
where ħ (h-bar) is the reduced Planck constant (h/2π). This equation demonstrates that the angular momentum is quantized; it can only take on specific discrete values determined by the integer value of l. A higher value of l corresponds to a larger orbital angular momentum. For example, an electron in a d orbital (l=2) has a larger angular momentum than an electron in a p orbital (l=1).
Subshells and Electron Configuration
The angular momentum quantum number is instrumental in understanding electron configurations and the periodic table. Each value of l represents a subshell within a principal energy level. These subshells are denoted by letters:
- l = 0: s subshell
- l = 1: p subshell
- l = 2: d subshell
- l = 3: f subshell
The number of orbitals within a subshell is determined by the magnetic quantum number (ml), which can have 2l + 1 values. Therefore:
- s subshell: 1 orbital
- p subshell: 3 orbitals
- d subshell: 5 orbitals
- f subshell: 7 orbitals
This directly impacts the electron capacity of each subshell; each orbital can hold a maximum of two electrons (due to the Pauli exclusion principle).
Implications for Chemical Bonding and Reactivity
The shape of the atomic orbitals, determined by l, has profound implications for chemical bonding and the reactivity of elements. For instance:
-
Directional Bonding: The directional nature of p and d orbitals leads to the formation of directional covalent bonds, where electrons are shared between atoms in specific directions. This is crucial in understanding the geometry of molecules.
-
Hybridization: The mixing of atomic orbitals (like s and p orbitals) to form hybrid orbitals with different shapes and energies is influenced by the shapes determined by l. This process is essential in explaining the bonding in organic molecules and other compounds.
-
Complex Ion Formation: The d orbitals are central to the formation of coordination complexes (or complex ions) in transition metal chemistry. The shapes and energy levels of the d orbitals dictate the geometry and properties of these complexes.
-
Spectroscopic Properties: The energy differences between orbitals with different l values contribute to the absorption and emission spectra of atoms and molecules, providing valuable information about their electronic structure.
The Role of l in Advanced Quantum Mechanics
The angular momentum quantum number plays a critical role in more advanced quantum mechanical treatments of atomic structure. For example:
-
Spin-Orbit Coupling: The interaction between the electron's spin angular momentum and its orbital angular momentum (dependent on l) leads to fine structure splitting in atomic spectra. This effect is particularly significant for heavier atoms.
-
Relativistic Effects: In heavier atoms, relativistic effects become more pronounced. These effects, which arise from the high speeds of electrons near the nucleus, can significantly alter the energies and shapes of orbitals, especially those with high l values.
Conclusion: A Cornerstone of Atomic Structure
The angular momentum quantum number (l) is not merely a label; it is a fundamental parameter that shapes our understanding of atomic structure and the properties of matter. It defines the shape of atomic orbitals, directly influencing electron distribution, bonding characteristics, and the chemical and physical behaviors of elements and their compounds. From the simple spherical symmetry of s orbitals to the complex shapes of f orbitals, l provides the essential framework for interpreting the intricate world of quantum mechanics and its impact on the macroscopic world we observe. A thorough understanding of l is indispensable for anyone seeking a deep comprehension of chemistry, physics, and materials science.
Latest Posts
Latest Posts
-
What Is The Percentage Of 17 20
Mar 18, 2025
-
Integral Of Sec X Tan X
Mar 18, 2025
-
How Many Ounces Are In A Quarter Gallon
Mar 18, 2025
-
What Are The Factors Of 43
Mar 18, 2025
-
Integral Of Square Root Of 4 X 2
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Which Characteristic Is Given By The Angular Momentum Quantum Number . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
