What Temperature Does Water Boil Kelvin
listenit
Mar 25, 2025 · 5 min read
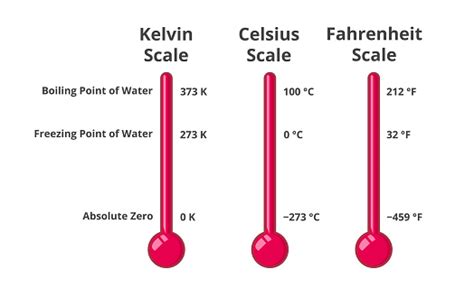
Table of Contents
What Temperature Does Water Boil in Kelvin? Understanding the Kelvin Scale and Boiling Points
The question, "What temperature does water boil in Kelvin?" seems simple, but it opens a door to a deeper understanding of temperature scales and the properties of water. While most are familiar with Celsius and Fahrenheit, the Kelvin scale plays a crucial role in scientific applications, particularly when dealing with the absolute zero point of temperature. This comprehensive guide will explore the boiling point of water in Kelvin, explain the Kelvin scale, delve into factors influencing boiling point, and examine related concepts.
Understanding the Kelvin Scale
Unlike Celsius and Fahrenheit, which are relative scales based on arbitrary reference points (freezing and boiling points of water), the Kelvin scale is an absolute temperature scale. This means its zero point, 0 Kelvin (0 K), represents absolute zero – the theoretical point where all molecular motion ceases. This fundamental difference makes the Kelvin scale particularly useful in scientific calculations and understanding thermodynamic processes.
Key Differences from Celsius and Fahrenheit:
- Absolute Zero: The Kelvin scale's defining characteristic is its starting point at absolute zero. This provides a consistent and universally understood base for thermodynamic measurements.
- No Negative Temperatures: Because the scale begins at absolute zero, there are no negative Kelvin temperatures. This simplifies many calculations and avoids the complexities associated with negative values in other scales.
- Relationship to Celsius: The Kelvin and Celsius scales are directly related. The size of a Kelvin degree is identical to the size of a Celsius degree. To convert Celsius to Kelvin, simply add 273.15: K = °C + 273.15.
The Boiling Point of Water in Kelvin
Water boils at 100°C at standard atmospheric pressure (1 atmosphere or 101.325 kPa). Using the conversion formula above, we can easily determine the boiling point of water in Kelvin:
K = 100°C + 273.15 = 373.15 K
Therefore, water boils at 373.15 Kelvin under standard atmospheric pressure. This is a crucial constant used in numerous scientific and engineering applications.
Factors Affecting Water's Boiling Point
While 373.15 K is the standard boiling point, it's important to remember that several factors can influence the temperature at which water boils:
1. Atmospheric Pressure:
This is arguably the most significant factor. Lower atmospheric pressure lowers the boiling point, and vice-versa. At higher altitudes, where atmospheric pressure is reduced, water boils at a lower temperature. This is why cooking times are often longer at higher elevations. Conversely, increasing pressure increases the boiling point. Pressure cookers utilize this principle to cook food faster at higher temperatures.
2. Impurities in Water:
Dissolved substances in water, such as salts or minerals, can slightly elevate the boiling point. This phenomenon is known as boiling point elevation. The extent of the elevation depends on the concentration of the dissolved impurities. While the effect is usually minimal for everyday applications, it's significant in scientific experiments requiring high precision.
3. Isotopic Composition:
The isotopic composition of water can also have a subtle impact on the boiling point. Water molecules containing heavier isotopes (like deuterium instead of regular hydrogen) will have a slightly higher boiling point than water composed of lighter isotopes. This effect is usually negligible in most contexts.
Applications of the Kelvin Scale and Water's Boiling Point
The Kelvin scale and the precise boiling point of water are fundamental to many scientific and engineering disciplines:
1. Thermodynamics:
The Kelvin scale is essential for thermodynamic calculations, particularly those involving heat transfer, work, and entropy. Understanding the absolute temperature of water's boiling point is crucial for analyzing phase transitions and thermodynamic equilibria.
2. Chemistry:
In chemistry, the boiling point of water in Kelvin is used in various calculations related to solutions, reactions, and phase diagrams. It helps determine reaction rates, equilibrium constants, and other crucial parameters.
3. Physics:
Physics applications range from studying the behavior of gases at different temperatures to analyzing heat engines and thermal processes. The Kelvin scale ensures consistency and accuracy in these calculations.
4. Engineering:
Engineers use the boiling point of water in designing systems involving heating, cooling, and steam generation. Understanding the influence of pressure on boiling point is crucial for designing efficient and safe systems.
5. Meteorology:
Meteorologists use temperature data, often expressed in Kelvin, to understand atmospheric processes, cloud formation, and weather patterns. The boiling point of water is a benchmark in understanding humidity and atmospheric stability.
Beyond Boiling: Understanding Phase Transitions
The boiling point of water marks the transition from the liquid to the gaseous phase. Understanding phase transitions is crucial in many scientific fields.
Phase Diagram of Water:
A phase diagram graphically represents the states of matter (solid, liquid, gas) of a substance as a function of temperature and pressure. The phase diagram of water shows the conditions under which water exists as ice, liquid water, or water vapor. The line separating the liquid and gas phases indicates the boiling point at different pressures.
Sublimation and Deposition:
Water can also undergo sublimation (transition from solid to gas) and deposition (transition from gas to solid) under specific conditions of temperature and pressure. These processes are less common than melting/freezing and boiling/condensation, but still crucial in understanding atmospheric phenomena like snow formation.
Conclusion: The Significance of 373.15 K
The boiling point of water at 373.15 Kelvin is far more than just a number. It's a fundamental constant in science and engineering, reflecting the interplay between temperature, pressure, and the molecular behavior of water. Understanding this point, and the factors that influence it, is crucial for diverse applications, from everyday cooking to advanced scientific research. The Kelvin scale, with its absolute zero point, provides a robust and consistent framework for measuring and interpreting temperature data, contributing to the advancement of scientific knowledge across various disciplines. As we continue to explore the complexities of the physical world, accurate measurement and understanding of fundamental constants like the boiling point of water in Kelvin remain indispensable. Further research into the behavior of water under extreme conditions, both in terms of pressure and temperature, will continue to refine our understanding of this vital substance and its critical role in our world.
Latest Posts
Latest Posts
-
3 Main Ideas Of Cell Theory
Mar 28, 2025
-
What Are The Most Reactive Metals In The Periodic Table
Mar 28, 2025
-
Is Supports Combustion A Physical Or Chemical Property
Mar 28, 2025
-
What Is 6 Divided By 1 3
Mar 28, 2025
-
Add Acid To Water Or Water To Acid
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Temperature Does Water Boil Kelvin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
