Add Acid To Water Or Water To Acid
listenit
Mar 28, 2025 · 5 min read
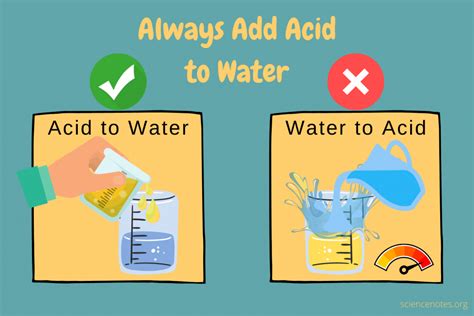
Table of Contents
Add Acid to Water or Water to Acid: A Comprehensive Guide to Safe Chemical Handling
The question of whether to add acid to water or water to acid is not merely a matter of curiosity; it's a fundamental safety precaution in any laboratory or industrial setting involving the handling of acids. The consequences of getting this wrong can be severe, ranging from minor burns to explosions. This comprehensive guide will delve into the science behind this crucial safety rule, explore the potential dangers, and provide practical advice for safe acid handling.
The Science Behind the Safety Rule
The key to understanding this safety precaution lies in the exothermic nature of acid dilution. When an acid is added to water, the acid molecules are surrounded by water molecules. This process generates heat. The amount of heat generated depends on the concentration and type of acid. For strong acids like sulfuric acid, this heat generation can be quite substantial.
Adding acid to water allows the heat generated to be dissipated safely across a large volume of water. The water acts as a heat sink, preventing a localized rise in temperature that could cause the acid to boil or splatter. The heat is absorbed by the water, leading to a gradual increase in temperature.
The Dangers of Adding Water to Acid
Conversely, adding water to acid leads to a drastically different outcome. When water is added to a concentrated acid, the heat generated is concentrated in a smaller volume of water. This localized heating can lead to several dangerous consequences:
1. Boiling and Spattering:
The rapid heat generation can cause the water to boil almost instantly. This boiling action results in violent spattering of the concentrated acid, which can cause significant burns and injuries. The spattered acid can also contaminate the surrounding area, presenting a further hazard.
2. Explosive Reactions:
In some cases, particularly with highly concentrated acids like sulfuric acid, adding water can lead to an explosive reaction. The heat generated is so intense that it can cause the acid to vaporize rapidly, creating a pressure build-up that can lead to a violent explosion. The resulting shrapnel of glass and acid can cause severe injuries.
3. Uncontrolled Temperature Increase:
The lack of sufficient water to absorb the heat leads to an uncontrolled temperature increase. This can reach temperatures high enough to ignite flammable materials in the vicinity, leading to a fire or even an explosion.
Practical Safety Measures for Acid Dilution
Safe acid dilution requires careful planning and execution. Here's a step-by-step guide:
1. Personal Protective Equipment (PPE):
Before beginning any acid dilution procedure, always wear appropriate PPE. This includes:
- Safety Goggles: To protect your eyes from splashes and fumes.
- Lab Coat: To protect your clothing from acid spills.
- Gloves: Chemical-resistant gloves made of materials like nitrile or neoprene are essential.
- Closed-Toe Shoes: To protect your feet.
2. Choosing the Right Apparatus:
Use appropriate glassware that is resistant to the acid being diluted. Beakers, flasks, or graduated cylinders made of borosilicate glass (Pyrex) are commonly used. Avoid using cracked or chipped glassware. Consider using a heat-resistant container, especially for highly exothermic reactions.
3. Slow and Steady Dilution:
Always add the acid slowly and carefully to the water. A small stream or drops are recommended. Never pour large quantities of acid at once. Stir the solution gently but continuously to ensure even mixing and heat distribution.
4. Ice Bath (Optional):
For highly exothermic reactions, an ice bath can be used to help control the temperature increase. Place the reaction vessel in an ice bath before beginning the dilution process. Monitor the temperature closely using a thermometer.
5. Appropriate Ventilation:
Work under a well-ventilated area or use a fume hood to minimize exposure to acid fumes. Acid fumes can be irritating and corrosive.
6. Neutralization and Waste Disposal:
After the dilution is complete, carefully neutralize any remaining acid according to your institution's safety guidelines. Never pour acid down the drain without proper neutralization and disposal procedures. Follow all relevant environmental regulations for acid waste disposal.
Common Acids and Their Dilution Procedures
The dilution procedure may vary slightly depending on the specific acid being used. Here are some examples:
1. Sulfuric Acid (H₂SO₄):
Sulfuric acid is a highly corrosive and exothermic acid. Extreme caution must be taken when diluting sulfuric acid. Always add the acid to the water slowly and stir continuously. Use an ice bath if necessary to control the temperature.
2. Hydrochloric Acid (HCl):
Hydrochloric acid is also a strong acid, but generally less exothermic than sulfuric acid. However, it still requires careful dilution, following the same principles of adding acid to water slowly and stirring continuously.
3. Nitric Acid (HNO₃):
Nitric acid is a strong oxidizing acid. Dilution procedures should follow the same safety precautions as sulfuric and hydrochloric acid. Always add the acid to the water slowly and stir gently.
4. Acetic Acid (CH₃COOH):
Acetic acid (vinegar) is a weak acid and is generally less hazardous to dilute. However, it's still important to follow basic safety precautions such as wearing appropriate PPE and avoiding direct contact.
Understanding the "Why" Behind the Safety Rule
The underlying principle behind adding acid to water is to maximize the surface area of the water exposed to the acid. This maximizes the heat dissipation, preventing localized heating and minimizing the risk of boiling, spattering, and explosions. Adding water to acid concentrates the heat in a smaller volume, resulting in a potentially dangerous scenario.
Beyond the Basics: Advanced Considerations
For large-scale dilutions or highly concentrated acids, additional safety considerations should be implemented:
- Automated Dilution Systems: For industrial settings, automated dilution systems can help ensure safety and consistency.
- Temperature Monitoring: Constant temperature monitoring using sensors and alarms can help prevent uncontrolled temperature increases.
- Emergency Procedures: Having clear emergency procedures in place for acid spills or accidents is critical.
- Safety Training: Proper training for all personnel involved in handling acids is absolutely necessary.
Conclusion
The seemingly simple question of whether to add acid to water or water to acid has profound implications for safety. By understanding the science behind the safety rule, employing appropriate safety measures, and following established procedures, we can significantly reduce the risk of accidents and ensure the safe handling of acids in any setting. Remember, always add acid to water, never water to acid. This simple rule can prevent serious injury or even death. Prioritizing safety is not just a best practice; it is a necessity.
Latest Posts
Latest Posts
-
What Is The Name Of This Hydrocarbon
Mar 31, 2025
-
Columns Of Periodic Table Are Called
Mar 31, 2025
-
Difference Between Animal Mitosis And Plant Mitosis
Mar 31, 2025
-
What Is Half Of One And A Half Teaspoons
Mar 31, 2025
-
What Is 4 12 In Simplest Form
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Add Acid To Water Or Water To Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
