What Are The Most Reactive Metals In The Periodic Table
listenit
Mar 28, 2025 · 6 min read
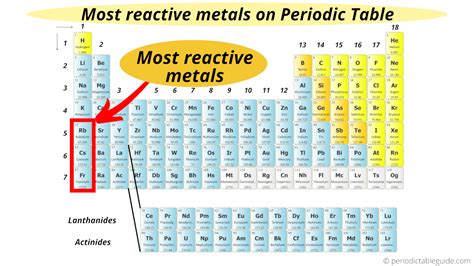
Table of Contents
- What Are The Most Reactive Metals In The Periodic Table
- Table of Contents
- What are the Most Reactive Metals in the Periodic Table?
- Understanding Metallic Reactivity
- 1. Ionization Energy:
- 2. Electronegativity:
- 3. Atomic Radius:
- 4. Shielding Effect:
- The Alkali Metals: The Most Reactive Group
- Lithium (Li):
- Sodium (Na):
- Potassium (K):
- Rubidium (Rb):
- Cesium (Cs):
- Francium (Fr):
- Alkaline Earth Metals: A Less Reactive, but Still Significant Group
- Other Reactive Metals
- Applications of Reactive Metals
- Safety Considerations
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
What are the Most Reactive Metals in the Periodic Table?
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. One crucial property is reactivity, which describes how readily an element undergoes chemical reactions. Among the elements, metals stand out for their varied and often vigorous reactions. This article delves deep into the world of reactive metals, exploring the factors that govern their reactivity and highlighting the most reactive metals found in the periodic table. We'll explore their properties, reactions, and applications, explaining why they are so important in various fields.
Understanding Metallic Reactivity
The reactivity of a metal is fundamentally linked to its electronic configuration, specifically the ease with which it loses electrons. Metals, by their nature, tend to have relatively low electronegativity – meaning they don't strongly attract electrons. This makes them eager to donate their valence electrons (the outermost electrons) to achieve a more stable electron configuration, often resembling that of a noble gas. The easier it is for a metal to lose electrons, the more reactive it is.
Several factors influence a metal's reactivity:
1. Ionization Energy:
Ionization energy is the energy required to remove an electron from a neutral atom. Metals with low ionization energies readily lose electrons, exhibiting high reactivity. The lower the ionization energy, the easier it is to remove an electron and thus, the higher the reactivity.
2. Electronegativity:
As mentioned before, metals possess low electronegativity. Lower electronegativity means a weaker pull on electrons, leading to an increased tendency to lose electrons and participate in reactions.
3. Atomic Radius:
The atomic radius, or the size of an atom, plays a role. Larger atoms generally have weaker holds on their outermost electrons, resulting in higher reactivity. The further away the valence electrons are from the nucleus, the less strongly they are attracted, and hence, easier to lose.
4. Shielding Effect:
The inner electrons shield the outer electrons from the positive charge of the nucleus. Increased shielding reduces the effective nuclear charge experienced by valence electrons, making them easier to remove and increasing reactivity.
The Alkali Metals: The Most Reactive Group
The alkali metals (Group 1), located in the first column of the periodic table, are renowned for their exceptional reactivity. This stems from their electronic configuration: they all have one valence electron readily available for donation. As you move down the group, reactivity increases due to increasing atomic size and decreasing ionization energy.
Let's examine some key members of this highly reactive group:
Lithium (Li):
While the least reactive alkali metal, lithium still reacts vigorously with water, producing hydrogen gas and lithium hydroxide. Its reactivity is noticeably lower than other alkali metals because of its small size and relatively high ionization energy compared to its heavier counterparts.
Sodium (Na):
Sodium is considerably more reactive than lithium. Its reaction with water is quite dramatic, producing a significant amount of heat and hydrogen gas, often igniting the hydrogen. This reaction is often demonstrated in chemistry classes, showcasing the alkali metal's energetic nature.
Potassium (K):
Potassium exhibits even greater reactivity than sodium. Its reaction with water is even more vigorous, often resulting in a spontaneous flame. The increased reactivity is attributed to its larger size and lower ionization energy.
Rubidium (Rb):
Rubidium is significantly more reactive than potassium. Its reaction with water is extremely vigorous, producing a substantial amount of heat and often leading to an explosion. The extreme reactivity necessitates handling it under strictly controlled conditions.
Cesium (Cs):
Cesium holds the title of the most reactive alkali metal. Its reaction with water is explosive and extremely rapid. Even exposure to air can trigger a reaction, emphasizing the need for meticulous handling and storage under inert atmospheres. The extreme reactivity is a direct consequence of its very large atomic size, the lowest ionization energy, and the weakest hold on its valence electron among all the alkali metals.
Francium (Fr):
Francium, a radioactive element, theoretically possesses the highest reactivity among all elements. However, due to its extreme rarity and radioactive nature, experimental verification of its reactivity is limited. Its predicted properties based on its position in the periodic table suggest an even more vigorous reaction with water than cesium.
Alkaline Earth Metals: A Less Reactive, but Still Significant Group
The alkaline earth metals (Group 2) are less reactive than the alkali metals because they have two valence electrons instead of one. While they still readily lose electrons, the process requires more energy than for alkali metals. Nevertheless, they are still considered highly reactive.
The reactivity within this group also increases down the periodic table:
- Magnesium (Mg): Magnesium reacts with hot water and acids, although not as violently as alkali metals.
- Calcium (Ca): Calcium reacts more vigorously with water than magnesium.
- Strontium (Sr): Strontium shows even greater reactivity than calcium.
- Barium (Ba): Barium is the most reactive alkaline earth metal, reacting readily with cold water.
- Radium (Ra): Like Francium, Radium is a radioactive element and its reactivity is extremely high, but limited experimental data is available due to its radioactivity and scarcity.
Other Reactive Metals
Beyond the alkali and alkaline earth metals, several other metals exhibit notable reactivity:
- Aluminum (Al): While possessing a protective oxide layer that hinders reaction, aluminum reacts readily with strong acids and bases.
- Zinc (Zn): Zinc reacts with acids and bases to form salts.
- Iron (Fe): Iron, while not as reactive as the alkali metals, is susceptible to corrosion (rusting) due to its reaction with oxygen and water.
Applications of Reactive Metals
The high reactivity of these metals, while posing handling challenges, also makes them valuable in diverse applications:
- Lithium-ion batteries: Lithium's high reactivity and low atomic weight make it a crucial component in lithium-ion batteries, powering countless electronic devices.
- Sodium lamps: Sodium's reactivity is harnessed in sodium vapor lamps, which produce bright yellow light.
- Potassium in fertilizers: Potassium is an essential nutrient for plants and is used extensively in fertilizers.
- Calcium in construction: Calcium compounds are used in construction materials like cement.
- Magnesium alloys: Magnesium's relatively low density and high strength make its alloys valuable in aerospace and automotive applications.
Safety Considerations
Working with highly reactive metals requires extreme caution due to the potential for vigorous and sometimes dangerous reactions:
- Appropriate safety equipment: Protective gloves, goggles, and lab coats are essential.
- Controlled environments: Reactions should be conducted under controlled conditions to minimize risks.
- Inert atmospheres: Reactive metals are often stored and handled under inert gases like argon or nitrogen to prevent reactions with air.
- Proper disposal: Waste products must be handled safely and disposed of according to regulations.
Conclusion
The reactivity of metals is a fascinating aspect of chemistry, underpinned by their electronic configurations and influenced by factors like ionization energy, electronegativity, atomic radius, and shielding effects. The alkali metals, particularly cesium and francium, stand out as the most reactive, exhibiting dramatic reactions with water and air. Understanding the reactivity of metals is crucial not only for safe handling but also for harnessing their unique properties in various applications, from energy storage to construction and beyond. Further research continues to explore the intricacies of metallic reactivity, expanding our understanding of these essential elements and their roles in our world.
Latest Posts
Latest Posts
-
What Is 2 4 In A Fraction
Mar 31, 2025
-
Organisms That Cannot Make Their Own Food
Mar 31, 2025
-
How Many Electrons Are In The 4th Energy Level
Mar 31, 2025
-
How To Convert Molarity To Molality
Mar 31, 2025
-
How Much Is 1000 Feet In Miles
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Are The Most Reactive Metals In The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
