How To Convert Molarity To Molality
listenit
Mar 31, 2025 · 5 min read
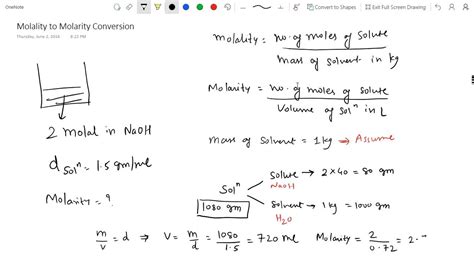
Table of Contents
How to Convert Molarity to Molality: A Comprehensive Guide
Molarity and molality are both crucial concentration units in chemistry, expressing the amount of solute dissolved in a solvent. However, they differ significantly in how they define concentration. Understanding the difference and mastering the conversion between them is vital for accurate calculations and a deep understanding of solution chemistry. This comprehensive guide will walk you through the process of converting molarity to molality, providing step-by-step instructions, examples, and crucial considerations.
Understanding Molarity and Molality
Before diving into the conversion, let's solidify our understanding of each concentration unit.
Molarity (M)
Molarity is defined as the number of moles of solute per liter of solution. The formula is:
Molarity (M) = moles of solute / liters of solution
It's a convenient unit because it directly relates the amount of solute to the total volume of the solution, readily measured in a laboratory. However, molarity is temperature-dependent because the volume of a solution changes with temperature.
Molality (m)
Molality, on the other hand, is defined as the number of moles of solute per kilogram of solvent. The formula is:
Molality (m) = moles of solute / kilograms of solvent
Unlike molarity, molality is temperature-independent because the mass of the solvent remains constant regardless of temperature changes. This makes molality particularly useful in situations where temperature fluctuations are significant, such as in colligative property calculations.
The Conversion Process: Molarity to Molality
Converting molarity to molality requires a few key steps and utilizes the relationship between the volume of the solution and the mass of the solvent. Here’s a breakdown of the process:
1. Understanding the Missing Link: Density
The critical piece of information needed to convert molarity to molality is the density of the solution. Density (ρ) is defined as the mass per unit volume, typically expressed in g/mL or kg/L. The density connects the volume of the solution (used in molarity) to the mass of the solution.
2. Calculating the Mass of the Solution
Once you have the density (ρ) and the volume of the solution (V), you can calculate the mass of the solution (m<sub>solution</sub>) using the following formula:
m<sub>solution</sub> = ρ × V
Remember to ensure consistent units; if your density is in g/mL, your volume should be in mL. If your density is in kg/L, your volume should be in L.
3. Determining the Mass of the Solvent
This is where the subtle difference between molarity and molality becomes crucial. We need to find the mass of the solvent and not the mass of the solution. To do this, we need to subtract the mass of the solute from the mass of the solution.
First, we calculate the mass of the solute (m<sub>solute</sub>) using the number of moles (n) and the molar mass (M<sub>m</sub>) of the solute:
m<sub>solute</sub> = n × M<sub>m</sub>
Then, we subtract this from the mass of the solution to find the mass of the solvent (m<sub>solvent</sub>):
m<sub>solvent</sub> = m<sub>solution</sub> - m<sub>solute</sub>
4. Calculating the Molality
Finally, we can calculate the molality (m) using the number of moles of solute (n) and the mass of the solvent (m<sub>solvent</sub>) in kilograms:
Molality (m) = moles of solute (n) / mass of solvent (m<sub>solvent</sub> in kg)
Illustrative Examples
Let's work through a few examples to solidify the conversion process.
Example 1: Simple Conversion
A solution of sodium chloride (NaCl) has a molarity of 1.5 M and a density of 1.05 g/mL. Calculate the molality of the solution. Assume the volume of the solution is 1 L (1000 mL).
Solution:
-
Mass of the solution: m<sub>solution</sub> = ρ × V = 1.05 g/mL × 1000 mL = 1050 g = 1.05 kg
-
Moles of NaCl: Since the molarity is 1.5 M and the volume is 1 L, we have 1.5 moles of NaCl.
-
Mass of NaCl: m<sub>NaCl</sub> = n × M<sub>m</sub> = 1.5 mol × 58.44 g/mol ≈ 87.66 g
-
Mass of water: m<sub>water</sub> = m<sub>solution</sub> - m<sub>NaCl</sub> = 1050 g - 87.66 g ≈ 962.34 g = 0.96234 kg
-
Molality: m = moles of NaCl / mass of water (kg) = 1.5 mol / 0.96234 kg ≈ 1.56 m
Example 2: More Complex Scenario
A 2.0 M solution of sulfuric acid (H₂SO₄) has a density of 1.14 g/mL. Calculate its molality. Let's assume we're working with 1 L of solution.
Solution:
-
Mass of the solution: m<sub>solution</sub> = ρ × V = 1.14 g/mL × 1000 mL = 1140 g = 1.14 kg
-
Moles of H₂SO₄: Since the molarity is 2.0 M and the volume is 1 L, we have 2.0 moles of H₂SO₄.
-
Mass of H₂SO₄: m<sub>H₂SO₄</sub> = n × M<sub>m</sub> = 2.0 mol × 98.08 g/mol ≈ 196.16 g
-
Mass of water: m<sub>water</sub> = m<sub>solution</sub> - m<sub>H₂SO₄</sub> = 1140 g - 196.16 g ≈ 943.84 g = 0.94384 kg
-
Molality: m = moles of H₂SO₄ / mass of water (kg) = 2.0 mol / 0.94384 kg ≈ 2.12 m
Considerations and Limitations
While the conversion process is straightforward, there are some crucial considerations:
-
Accuracy of Density: The accuracy of the molality calculation heavily relies on the accuracy of the density measurement. Inaccurate density measurements will lead to inaccurate molality values.
-
Ideal Solutions: The calculations assume the solution behaves ideally. For concentrated solutions or solutions with strong solute-solvent interactions, deviations from ideal behavior may occur, affecting the accuracy of the conversion.
-
Units: Maintaining consistent units throughout the calculation is essential to prevent errors.
-
Temperature Dependence of Density: Remember that density is temperature-dependent. Specify the temperature at which the density measurement was taken.
Conclusion
Converting molarity to molality is a fundamental skill in chemistry, offering a deeper understanding of solution concentrations and their behavior under varying conditions. This guide provides a comprehensive step-by-step approach, highlighting the importance of density and providing illustrative examples to help you master the conversion. By understanding the nuances and limitations of the process, you can perform these calculations with confidence and accuracy, contributing to your overall understanding of solution chemistry. Remember to always double-check your units and consider the potential limitations associated with non-ideal solutions. With practice, this conversion will become second nature, empowering you to tackle more complex chemical problems.
Latest Posts
Latest Posts
-
What Happens To Pressure When Volume Increases
Apr 02, 2025
-
What Is Lcm Of 10 And 12
Apr 02, 2025
-
25 Of What Number Is 6
Apr 02, 2025
-
What Is The Circumference Of A Circle With Radius 3
Apr 02, 2025
-
36 Is What Percent Of 45
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How To Convert Molarity To Molality . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
