What State Of Matter Has The Most Kinetic Energy
listenit
Mar 30, 2025 · 5 min read
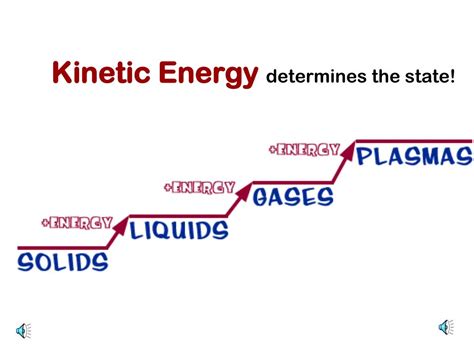
Table of Contents
What State of Matter Has the Most Kinetic Energy?
The question of which state of matter possesses the most kinetic energy isn't a simple one with a single, universally applicable answer. It depends heavily on the specific conditions, including temperature and pressure. However, we can explore the relationships between kinetic energy, temperature, and the different states of matter (solid, liquid, gas, and plasma) to gain a deeper understanding. This exploration will involve delving into the microscopic behavior of particles and how that translates to macroscopic properties like temperature and kinetic energy.
Understanding Kinetic Energy and States of Matter
Before we delve into the comparison, let's establish a clear understanding of the fundamental concepts.
Kinetic energy is the energy an object possesses due to its motion. At the microscopic level, this means the energy associated with the movement of atoms and molecules. The faster the particles move, the higher their kinetic energy.
The states of matter – solid, liquid, gas, and plasma – represent different levels of particle organization and interaction.
-
Solids: Particles in solids are tightly packed and have strong intermolecular forces holding them together in a fixed structure. Their movement is restricted to vibrations around fixed points. Kinetic energy is relatively low.
-
Liquids: Particles in liquids are closer together than in gases but further apart than in solids. Intermolecular forces are weaker than in solids, allowing for more movement and less fixed structure. Particles can flow and change positions. Kinetic energy is higher than in solids.
-
Gases: Particles in gases are widely dispersed and have very weak intermolecular forces. They move freely and randomly at high speeds, colliding frequently with each other and the container walls. Kinetic energy is significantly higher than in liquids and solids.
-
Plasma: Plasma is often considered the fourth state of matter. It's a highly energized state where electrons are stripped from atoms, resulting in a mixture of ions and free electrons. Particles in plasma move at extremely high speeds, leading to very high kinetic energy.
Comparing Kinetic Energy Across States of Matter at a Given Temperature
At a given temperature, the average kinetic energy of particles is directly proportional to the temperature. This means that at the same temperature, particles in all states of matter will have the same average kinetic energy. This is a crucial point often misunderstood. While the total kinetic energy might differ due to the number of particles, the average kinetic energy per particle is the same.
However, this is only true if we are considering the average kinetic energy. Individual particles within a given state will have varying kinetic energies. Some will be moving faster, others slower, due to random collisions and interactions.
The Role of Temperature and Pressure
Temperature plays a crucial role in determining the kinetic energy of particles. As temperature increases, the average kinetic energy of particles increases, regardless of the state of matter. This increased kinetic energy leads to a change in the state of matter.
-
Solid to Liquid (Melting): As the temperature of a solid increases, the particles gain enough kinetic energy to overcome the strong intermolecular forces holding them in place. This results in the solid melting into a liquid.
-
Liquid to Gas (Boiling/Vaporization): Further increases in temperature provide particles in a liquid with enough kinetic energy to overcome the remaining intermolecular forces and escape into the gaseous phase.
-
Gas to Plasma (Ionization): At extremely high temperatures, particles in a gas gain so much kinetic energy that electrons are stripped from atoms, forming plasma.
Pressure also affects the kinetic energy indirectly. Increased pressure forces particles closer together, increasing the frequency of collisions and thus influencing the distribution of kinetic energies among the particles. However, at a given temperature, the average kinetic energy will still be the same.
Total Kinetic Energy: A Different Perspective
While average kinetic energy is equal at a given temperature, the total kinetic energy is dependent on the number of particles present. A large volume of gas at a given temperature will possess more total kinetic energy than a small volume of solid at the same temperature simply because it contains far more particles.
Let's consider an example: A cubic meter of steam (gas) at 100°C will have far more total kinetic energy than a cubic meter of ice (solid) at 0°C, even though the average kinetic energy of the individual water molecules might be higher in the ice due to stronger intermolecular forces. This is because the steam contains a significantly larger number of particles.
The Exceptional Case of Plasma
Plasma presents a unique situation. While the average kinetic energy of individual particles in a plasma at a particular temperature might not be inherently higher than in a gas at the same temperature, the total kinetic energy can be substantially greater. This is due to the much higher energy involved in ionizing the atoms and maintaining the plasma state, which often requires significant external energy input. The extremely high speeds of ions and electrons contribute to the overall high kinetic energy.
Conclusion: Context is Key
The question of which state of matter possesses the most kinetic energy requires careful consideration of the context. At a given temperature, the average kinetic energy of particles is the same across all states of matter. However, the total kinetic energy can differ significantly due to differences in the number of particles and the energy involved in maintaining the state (especially in the case of plasma). A large volume of gas or plasma at a high temperature will generally possess the most total kinetic energy. The key takeaway is that while temperature directly impacts average kinetic energy, the number of particles and the energy required to sustain the state play crucial roles in determining the overall kinetic energy. Therefore, there's no single definitive answer without specifying the conditions. Understanding both average and total kinetic energy is essential for a complete understanding of the relationship between kinetic energy and states of matter.
Latest Posts
Latest Posts
-
Which Element Is Found In All Organic Compounds
Apr 01, 2025
-
Is Supporting Combustion A Physical Property
Apr 01, 2025
-
100 Cm Equals How Many Meters
Apr 01, 2025
-
Write The Equilibrium Constant Expression For This Reaction
Apr 01, 2025
-
What Is 3 6 In A Fraction
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What State Of Matter Has The Most Kinetic Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
