Is Supporting Combustion A Physical Property
listenit
Apr 01, 2025 · 5 min read
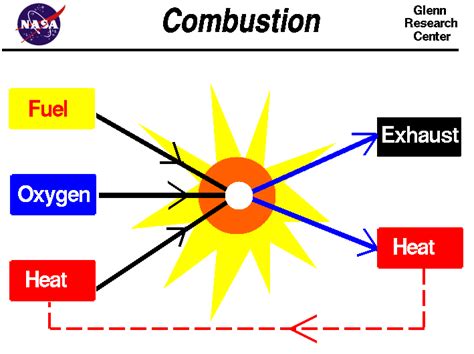
Table of Contents
Is Supporting Combustion a Physical Property? A Deep Dive into Chemical and Physical Properties
The question of whether supporting combustion is a physical or chemical property often sparks debate. While it might seem straightforward, a nuanced understanding requires exploring the definitions of both physical and chemical properties, examining the process of combustion itself, and considering the implications of this classification. This comprehensive article will delve into these aspects, providing a definitive answer supported by scientific evidence.
Understanding Physical and Chemical Properties
Before tackling the central question, let's establish clear definitions:
Physical properties are characteristics that can be observed or measured without changing the substance's chemical composition. These include properties like color, density, melting point, boiling point, and solubility. Crucially, changes in physical properties do not result in the formation of new substances. You can change the shape of a piece of gold, melt it, or dissolve it, but it remains gold.
Chemical properties, on the other hand, describe a substance's ability to undergo a chemical change, resulting in the formation of new substances with different compositions. Examples include flammability, reactivity with acids, and oxidation states. These properties are observed only when a substance undergoes a chemical reaction.
The Combustion Process: A Chemical Transformation
Combustion, often referred to as burning, is a rapid chemical reaction between a substance (the fuel) and an oxidant (usually oxygen), releasing energy in the form of heat and light. This process involves the breaking and formation of chemical bonds, leading to the creation of entirely new substances. For instance, the combustion of methane (CH₄) in oxygen (O₂) produces carbon dioxide (CO₂) and water (H₂O):
CH₄ + 2O₂ → CO₂ + 2H₂O + Heat + Light
This equation clearly illustrates a chemical transformation. The reactants, methane and oxygen, are fundamentally different from the products, carbon dioxide and water. The energy released is a consequence of the rearrangement of atoms and the formation of stronger bonds in the products. The original substances are consumed and replaced by new ones.
Supporting Combustion: A Chemical Property
The ability of a substance to support combustion directly relates to its capacity to act as an oxidant in a combustion reaction. Oxygen, the most common oxidant, readily participates in combustion reactions. However, other substances can also act as oxidants, albeit often under specific conditions. Examples include fluorine (F₂), chlorine (Cl₂), and certain nitrogen oxides.
Since supporting combustion necessitates a chemical reaction – the oxidation of the fuel – it inherently falls under the category of a chemical property. A substance's ability to support combustion reflects its chemical reactivity and its potential to undergo redox reactions (reduction-oxidation reactions) with fuels. It is not a characteristic observable without undergoing a chemical change. The substance's inherent chemical composition dictates its potential to participate in such reactions.
Examining the Arguments Against Classification as a Chemical Property
Some might argue that the physical presence of an oxidant is sufficient for combustion to occur. They might claim that the mere existence of oxygen near a flammable material is a physical property, and its involvement in combustion is a secondary consequence. This reasoning is flawed because it neglects the fundamental essence of combustion. While the presence of oxygen is a prerequisite, it's the interaction with the fuel, leading to chemical transformation, that defines combustion. The presence of oxygen is a necessary condition, but not a sufficient condition to declare it a physical property involved in combustion.
Another argument might center around the physical process of oxygen diffusion in the combustion process. However, the diffusion of oxygen is a physical process that precedes the chemical reaction of combustion. The chemical reaction itself is independent of the method of oxygen delivery. Diffusion, while essential for the reaction to occur, does not define the fundamental nature of the combustion process.
Analogies to Further Illustrate the Point
Consider the following analogies:
-
Water dissolving salt: Water's ability to dissolve salt is a physical property. The salt's chemical composition remains unchanged; it's simply dispersed within the water. The process is reversible. Combustion, in contrast, is irreversible and generates new chemical species.
-
Iron rusting: Iron rusting (oxidation) is a chemical property. The iron reacts with oxygen and water to form iron oxide, a completely different substance. Supporting combustion shares this fundamental characteristic of chemical transformation.
-
Paper burning: The paper's flammability is a chemical property. When ignited, it undergoes a chemical change, forming ash and gases. The presence of oxygen (supporting combustion) is crucial, but it's the chemical reaction resulting in new substances that identifies the property as chemical.
Practical Implications of Correct Classification
Correctly identifying supporting combustion as a chemical property is crucial in several fields:
-
Fire safety: Understanding the chemical properties of materials allows for better fire prevention and suppression strategies. Knowing what substances support combustion is vital for designing safe environments and developing effective fire extinguishing agents.
-
Material science: The ability of a material to support or resist combustion influences material selection in various applications, from construction to aerospace engineering.
-
Chemical engineering: Combustion processes are essential in numerous industrial applications, such as power generation and chemical synthesis. Precise control of combustion relies on a deep understanding of the chemical properties involved.
Conclusion: A Definitive Answer
In conclusion, supporting combustion is unequivocally a chemical property. While the physical presence of an oxidant is a prerequisite for combustion, the process itself involves a fundamental chemical change – the oxidation of a fuel, leading to the formation of new substances with different chemical compositions. The energy released is a direct consequence of this chemical transformation. Therefore, classifying the ability of a substance to support combustion as anything other than a chemical property is scientifically inaccurate and misrepresents the fundamental nature of this important chemical process. Understanding this distinction is critical for advancing various scientific and engineering disciplines.
Latest Posts
Latest Posts
-
How Many Grams In 8 Kilograms
Apr 02, 2025
-
12x 4y 20 Solve For Y
Apr 02, 2025
-
Most Reactive Group On The Periodic Table
Apr 02, 2025
-
How To Solve Multi Step Inequalities
Apr 02, 2025
-
Percent Composition Of Mg No3 2
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Is Supporting Combustion A Physical Property . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
