Write The Equilibrium Constant Expression For This Reaction
listenit
Apr 01, 2025 · 6 min read
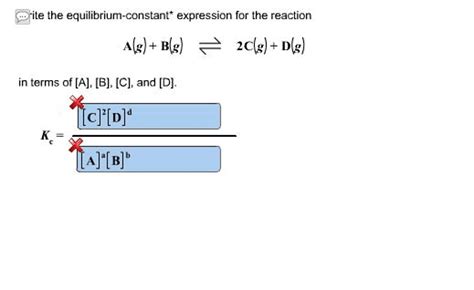
Table of Contents
Writing the Equilibrium Constant Expression: A Comprehensive Guide
Understanding and writing the equilibrium constant expression is fundamental to chemical equilibrium, a cornerstone of chemistry. This comprehensive guide will walk you through the process, covering various reaction types and complexities. We'll delve into the nuances of equilibrium constants, exploring their significance and applications in predicting reaction behavior. By the end, you'll be confidently crafting equilibrium constant expressions for a wide array of chemical reactions.
What is the Equilibrium Constant?
Before diving into the mechanics of writing expressions, let's clarify the concept of the equilibrium constant (K). It's a numerical value that describes the ratio of products to reactants at equilibrium for a reversible reaction at a specific temperature. A reversible reaction is one that proceeds in both the forward and reverse directions simultaneously. Crucially, the equilibrium constant doesn't change unless the temperature alters.
The value of K provides valuable insights into the reaction:
- K >> 1 (K > 10<sup>3</sup>): The reaction strongly favors product formation at equilibrium. The equilibrium lies far to the right.
- K ≈ 1 (0.1 < K < 10): Significant amounts of both reactants and products are present at equilibrium. The equilibrium is relatively balanced.
- K << 1 (K < 10<sup>-3</sup>): The reaction strongly favors reactant formation at equilibrium. The equilibrium lies far to the left.
Writing the Equilibrium Constant Expression: The Law of Mass Action
The equilibrium constant expression is derived from the Law of Mass Action. This law states that at a given temperature, the ratio of the product of the activities or concentrations of the products to the product of the activities or concentrations of the reactants, each raised to the power of its stoichiometric coefficient, is constant.
For a general reversible reaction:
aA + bB ⇌ cC + dD
where a, b, c, and d are the stoichiometric coefficients, the equilibrium constant expression (K<sub>c</sub>, using concentrations) is:
K<sub>c</sub> = [C]<sup>c</sup>[D]<sup>d</sup> / [A]<sup>a</sup>[B]<sup>b</sup>
Let's break down the components:
- [A], [B], [C], [D]: Represent the equilibrium concentrations (in mol/L or M) of reactants A and B, and products C and D, respectively.
- a, b, c, d: Represent the stoichiometric coefficients (the numbers in front of each chemical species) in the balanced chemical equation. These are exponents in the equilibrium constant expression.
Important Note: Pure solids and pure liquids are not included in the equilibrium constant expression because their concentrations remain essentially constant throughout the reaction. Only the concentrations of aqueous species and gases are included.
Examples of Equilibrium Constant Expressions
Let's illustrate with several examples, showcasing different reaction types:
Example 1: Simple Reversible Reaction
Consider the reversible reaction:
N<sub>2</sub>(g) + 3H<sub>2</sub>(g) ⇌ 2NH<sub>3</sub>(g)
The equilibrium constant expression is:
K<sub>c</sub> = [NH<sub>3</sub>]<sup>2</sup> / [N<sub>2</sub>][H<sub>2</sub>]<sup>3</sup>
Example 2: Reaction Involving a Solid
Consider the decomposition of calcium carbonate:
CaCO<sub>3</sub>(s) ⇌ CaO(s) + CO<sub>2</sub>(g)
Since CaCO<sub>3</sub>(s) and CaO(s) are pure solids, they are omitted from the expression. Therefore:
K<sub>c</sub> = [CO<sub>2</sub>]
Example 3: Reaction with Multiple Products and Reactants
Consider the reaction:
2SO<sub>2</sub>(g) + O<sub>2</sub>(g) ⇌ 2SO<sub>3</sub>(g)
The equilibrium constant expression is:
K<sub>c</sub> = [SO<sub>3</sub>]<sup>2</sup> / [SO<sub>2</sub>]<sup>2</sup>[O<sub>2</sub>]
Example 4: Reaction with a Liquid Solvent (Water)
Consider the ionization of acetic acid in water:
CH<sub>3</sub>COOH(aq) + H<sub>2</sub>O(l) ⇌ CH<sub>3</sub>COO<sup>-</sup>(aq) + H<sub>3</sub>O<sup>+</sup>(aq)
Water (as the solvent) is in large excess and its concentration remains relatively constant. Therefore, it's omitted from the expression:
K<sub>c</sub> = [CH<sub>3</sub>COO<sup>-</sup>][H<sub>3</sub>O<sup>+</sup>] / [CH<sub>3</sub>COOH] This is often represented as K<sub>a</sub>, the acid dissociation constant.
K<sub>p</sub>: The Equilibrium Constant Using Partial Pressures
When dealing with gaseous reactions, it's often more convenient to express the equilibrium constant in terms of partial pressures (K<sub>p</sub>) rather than concentrations. For the general reaction:
aA(g) + bB(g) ⇌ cC(g) + dD(g)
K<sub>p</sub> = (P<sub>C</sub><sup>c</sup>P<sub>D</sub><sup>d</sup>) / (P<sub>A</sub><sup>a</sup>P<sub>B</sub><sup>b</sup>)
Where P<sub>A</sub>, P<sub>B</sub>, P<sub>C</sub>, and P<sub>D</sub> represent the partial pressures of the respective gases at equilibrium.
The relationship between K<sub>p</sub> and K<sub>c</sub> is given by:
K<sub>p</sub> = K<sub>c</sub>(RT)<sup>Δn</sup>
where:
- R is the ideal gas constant (0.0821 L·atm/mol·K)
- T is the temperature in Kelvin
- Δn is the change in the number of moles of gas (moles of gaseous products - moles of gaseous reactants)
Factors Affecting the Equilibrium Constant
While the equilibrium constant is independent of initial concentrations, it's crucial to remember its dependence on:
- Temperature: Changes in temperature directly affect the equilibrium constant. Increasing the temperature for an endothermic reaction (heat is a reactant) will increase K, while increasing the temperature for an exothermic reaction (heat is a product) will decrease K.
- Catalyst: Catalysts do not affect the equilibrium constant. They only speed up the rate at which equilibrium is reached.
Applications of Equilibrium Constants
Equilibrium constants find extensive applications in various fields:
- Predicting Reaction Direction: Knowing K allows predicting whether a reaction will proceed predominantly to the right (products) or left (reactants) under specific conditions.
- Calculating Equilibrium Concentrations: Using the equilibrium constant and the ICE (Initial, Change, Equilibrium) table, we can calculate the equilibrium concentrations of reactants and products.
- Solubility Product (K<sub>sp</sub>): This equilibrium constant is used to describe the solubility of sparingly soluble ionic compounds.
- Acid Dissociation Constant (K<sub>a</sub>) and Base Dissociation Constant (K<sub>b</sub>): These constants are used to quantify the strength of acids and bases.
Advanced Topics and Considerations
This guide provides a solid foundation for writing equilibrium constant expressions. However, more complex scenarios exist that require advanced techniques:
- Complex Ion Formation: The formation of complex ions involves multiple equilibria, requiring consideration of stepwise equilibrium constants.
- Heterogeneous Equilibria: These involve multiple phases (solid, liquid, gas) and require careful consideration of which components to include in the expression.
- Simultaneous Equilibria: Systems might involve multiple simultaneous equilibria, necessitating the solution of simultaneous equations to determine equilibrium concentrations.
Conclusion
Mastering the skill of writing equilibrium constant expressions is a critical step in understanding and predicting the behavior of chemical systems. This comprehensive guide has equipped you with the knowledge to tackle a broad range of reaction types. Remember to carefully balance the chemical equation, identify the appropriate components for inclusion in the expression (considering pure solids and liquids), and correctly apply the stoichiometric coefficients as exponents. With practice and a solid grasp of the underlying principles, you'll be able to confidently analyze and interpret chemical equilibrium. By applying the principles outlined here and consistently practicing, you'll develop a deep understanding of equilibrium constants and their critical role in chemistry. Remember to consult textbooks and other resources for further in-depth learning on specific advanced topics.
Latest Posts
Latest Posts
-
Natural Resources Of The Northeast Region
Apr 02, 2025
-
How Many Unpaired Electrons Are In Sulfur
Apr 02, 2025
-
Whats The Square Root Of 69
Apr 02, 2025
-
What Is The Least Common Multiple Of 10 And 15
Apr 02, 2025
-
How To Convert Ev To Joules
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Write The Equilibrium Constant Expression For This Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
