What Parts Of Atoms Are Involved In Chemical Reactions
listenit
Mar 23, 2025 · 6 min read
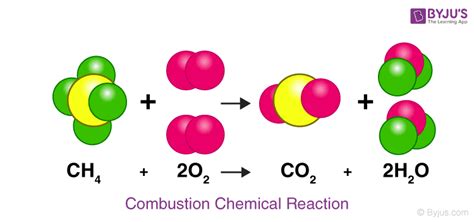
Table of Contents
What Parts of Atoms are Involved in Chemical Reactions?
Chemical reactions are the foundation of all changes we observe in the world around us, from the rusting of iron to the processes of life itself. But what exactly happens at the atomic level during these reactions? Understanding this involves delving into the structure of the atom and identifying the specific subatomic particles that play a crucial role. This article will explore the intricacies of atomic structure and explain how different parts of atoms contribute to chemical reactivity.
The Atom: A Brief Overview
Before diving into the specifics of chemical reactions, let's establish a basic understanding of atomic structure. An atom, the fundamental unit of matter, comprises three primary subatomic particles:
-
Protons: Positively charged particles residing in the atom's nucleus (the dense central core). The number of protons defines an element's atomic number and its unique identity on the periodic table.
-
Neutrons: Neutral particles also found within the nucleus. Their number can vary within the same element, leading to isotopes (atoms of the same element with different numbers of neutrons).
-
Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. These shells are not physical orbits but represent regions of probability where electrons are most likely to be found. The arrangement of electrons determines an atom's chemical behavior.
The Crucial Role of Electrons in Chemical Reactions
While protons and neutrons contribute to an atom's mass and stability, electrons are the primary players in chemical reactions. This is because electrons occupy the outermost shell, known as the valence shell. Electrons in the valence shell are called valence electrons. These electrons are loosely bound to the atom and are easily involved in interactions with other atoms.
Electron Configuration and Reactivity
The number of valence electrons an atom possesses dictates its reactivity. Atoms strive to achieve a stable electron configuration, often resembling the electron configuration of the nearest noble gas (Group 18 elements). Noble gases have full valence shells, making them exceptionally unreactive. This drive for stability underlies the formation of chemical bonds.
Atoms with nearly full or nearly empty valence shells are particularly reactive. They readily participate in chemical reactions to either gain, lose, or share electrons to achieve a stable octet (eight valence electrons) or a duet (two valence electrons for hydrogen and helium).
Types of Chemical Bonds: A Consequence of Electron Interactions
Chemical bonds are the forces that hold atoms together in molecules and compounds. The type of bond formed depends on how atoms interact with each other's valence electrons:
-
Ionic Bonds: These bonds are formed through the transfer of electrons from one atom to another. This transfer creates ions: positively charged cations (atoms that have lost electrons) and negatively charged anions (atoms that have gained electrons). The electrostatic attraction between these oppositely charged ions forms the ionic bond. For example, in sodium chloride (NaCl), sodium (Na) loses an electron to become a Na⁺ cation, and chlorine (Cl) gains an electron to become a Cl⁻ anion. The strong electrostatic attraction between these ions forms the ionic bond.
-
Covalent Bonds: These bonds involve the sharing of electrons between atoms. Atoms share electrons to achieve a stable electron configuration. Covalent bonds are common in molecules like water (H₂O) and methane (CH₄). In water, oxygen shares electrons with two hydrogen atoms, forming two covalent bonds. The shared electrons are attracted to both the oxygen and hydrogen nuclei, resulting in a relatively strong bond.
-
Metallic Bonds: This type of bonding occurs in metals. In metallic bonding, valence electrons are delocalized, meaning they are not associated with a specific atom but rather move freely throughout the metallic lattice. This "sea" of delocalized electrons accounts for the characteristic properties of metals, such as high electrical and thermal conductivity, malleability, and ductility.
The Role of Protons and Neutrons in Chemical Reactions: Indirect Influence
While electrons are the primary actors in chemical reactions, protons and neutrons play an indirect but significant role:
-
Protons determine the element's identity and chemical properties: The number of protons defines the element's atomic number, determining its position on the periodic table and influencing its reactivity. Elements with similar numbers of valence electrons exhibit similar chemical properties, as seen in groups or columns on the periodic table.
-
Neutrons influence isotopic behavior: Isotopes of the same element have the same number of protons but different numbers of neutrons. While the chemical behavior is largely determined by the number of protons and electrons, the different masses of isotopes can slightly affect reaction rates and equilibrium positions in certain cases (kinetic isotope effects). For example, deuterium (²H), a heavier isotope of hydrogen, reacts more slowly than ordinary hydrogen (¹H) in some reactions.
-
Nuclear reactions vs. chemical reactions: It’s crucial to differentiate between chemical reactions and nuclear reactions. Chemical reactions involve changes in the arrangement of electrons, whereas nuclear reactions involve changes in the nucleus (protons and neutrons). Nuclear reactions, such as radioactive decay or nuclear fusion, release vastly greater amounts of energy than chemical reactions and are governed by different principles.
Factors Influencing Chemical Reactivity Beyond Atomic Structure
The reactivity of an atom is not solely determined by its electron configuration. Several other factors influence how readily an atom participates in chemical reactions:
-
Electronegativity: This property measures an atom's tendency to attract electrons in a chemical bond. Atoms with high electronegativity tend to attract electrons strongly, leading to polar covalent bonds or ionic bonds.
-
Atomic size: Larger atoms have their valence electrons farther from the nucleus, making them less tightly bound and more easily lost or shared.
-
Ionization energy: The energy required to remove an electron from an atom. Atoms with low ionization energy readily lose electrons.
-
Electron affinity: The energy change associated with gaining an electron. Atoms with high electron affinity readily gain electrons.
-
Environmental factors: Temperature, pressure, and the presence of catalysts can significantly influence the rate and outcome of chemical reactions. Catalysts, for example, lower the activation energy required for a reaction to occur, thus increasing the reaction rate without being consumed themselves.
Conclusion: A Symphony of Subatomic Interactions
Chemical reactions are complex processes governed by the interactions of atoms' subatomic particles. While electrons are the primary players, influencing the formation of chemical bonds and driving reactivity, protons and neutrons contribute indirectly through their roles in determining the element's identity, mass, and overall stability. A comprehensive understanding of chemical reactions requires considering both the fundamental properties of atoms and the influence of environmental factors. By appreciating the intricate dance of electrons, protons, and neutrons, we gain a deeper understanding of the physical and chemical world around us. This knowledge forms the basis for advancements in various fields, from materials science and medicine to environmental science and energy production. The study of chemical reactions is a continuous exploration into the fundamental building blocks of matter and their remarkable interactions, constantly revealing new insights and fostering innovations.
Latest Posts
Latest Posts
-
What Is The Band Of Stability
Mar 25, 2025
-
What Is 4 To The 2 Power
Mar 25, 2025
-
Common Multiples Of 15 And 9
Mar 25, 2025
-
How Many Valence Electrons In F
Mar 25, 2025
-
What Is 1 4 As A Whole Number
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about What Parts Of Atoms Are Involved In Chemical Reactions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
