What Is The Band Of Stability
listenit
Mar 25, 2025 · 6 min read
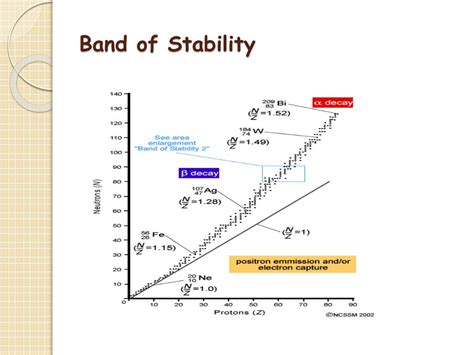
Table of Contents
What is the Band of Stability? Understanding Nuclear Stability and Isotopes
The band of stability is a crucial concept in nuclear physics and chemistry, describing the region on a chart of nuclides where stable atomic nuclei are found. Understanding this band is key to comprehending nuclear reactions, radioactive decay, and the behavior of elements. This article delves deep into the intricacies of the band of stability, exploring the factors that govern nuclear stability and the consequences of residing outside this region.
Understanding Nuclei: Protons, Neutrons, and the Strong Nuclear Force
At the heart of the band of stability lies the atomic nucleus, composed of protons and neutrons. Protons carry a positive charge, while neutrons are electrically neutral. The strong nuclear force, an incredibly powerful fundamental force, binds these nucleons (protons and neutrons) together, overcoming the electrostatic repulsion between the positively charged protons. The balance between this attractive strong force and the repulsive electromagnetic force dictates nuclear stability.
The Role of the Strong Nuclear Force
The strong nuclear force is short-ranged, meaning it only acts effectively over very short distances within the nucleus. This explains why larger nuclei are less stable – the repulsive forces between protons become more significant as the number of protons increases, exceeding the reach of the strong nuclear force for some nucleons. This is a key factor defining the limits of the band of stability.
The Neutron-to-Proton Ratio
A critical factor influencing nuclear stability is the neutron-to-proton ratio (N/Z ratio). For lighter elements (those with lower atomic numbers), a ratio of approximately 1:1 ensures stability. However, as the atomic number increases, the optimal N/Z ratio gradually increases to maintain stability. This is because the strong force is slightly stronger between neutron pairs compared to proton pairs. The extra neutrons help to counteract the increasing proton-proton repulsion in heavier nuclei.
The Chart of Nuclides: Visualizing Nuclear Stability
The chart of nuclides, also known as the Segrè chart, is a graphical representation of all known isotopes. It plots the number of neutrons (N) against the number of protons (Z) for each nuclide. Isotopes are atoms of the same element (same Z) but with different numbers of neutrons (different N). The band of stability is clearly visible on this chart as a region where stable nuclides cluster.
Identifying Stable and Unstable Nuclides
On the chart of nuclides, stable nuclides are represented by black squares, while unstable (radioactive) nuclides are shown in various colors depending on their decay mode (alpha, beta, etc.). Nuclides lying outside the band of stability are inherently unstable and will undergo radioactive decay to reach a more stable configuration within the band.
The Limits of the Band of Stability
The band of stability has well-defined limits. Beyond a certain number of protons (around 83, bismuth being the heaviest stable element), no stable isotopes exist. This is due to the overwhelming repulsive forces between protons in very heavy nuclei. The strong nuclear force can no longer overcome these forces, leading to inevitable radioactive decay.
Radioactive Decay: Returning to Stability
Nuclides located outside the band of stability undergo radioactive decay to achieve a more stable configuration. Several types of radioactive decay exist, each aiming to adjust the N/Z ratio and reduce the overall energy of the nucleus.
Alpha Decay
Alpha decay involves the emission of an alpha particle (a helium nucleus, consisting of two protons and two neutrons). This process reduces both the atomic number (Z) and mass number (A) of the nucleus, moving it closer to the band of stability. Alpha decay is common in heavy, unstable nuclei.
Beta Decay
Beta decay is a more complex process involving the conversion of a neutron into a proton (or vice versa). In beta-minus decay, a neutron converts into a proton, emitting an electron (beta particle) and an antineutrino. This increases the atomic number (Z) while decreasing the neutron number (N), again striving for a more stable N/Z ratio. Beta-plus decay involves the opposite conversion, transforming a proton into a neutron, emitting a positron (anti-electron) and a neutrino.
Gamma Decay
Gamma decay involves the emission of a gamma ray photon, a form of electromagnetic radiation. This process doesn't change the atomic number or mass number but reduces the energy of the nucleus, bringing it to a more stable energy state. Gamma decay often accompanies other types of decay.
Other Decay Modes
Other rarer decay modes exist, such as spontaneous fission (for extremely heavy nuclei) and electron capture (where a proton captures an inner-shell electron, converting into a neutron and emitting a neutrino). These processes also contribute to the nucleus finding its way back towards the band of stability.
Factors Influencing the Band of Stability: Shell Model and Pairing Effects
The simple neutron-to-proton ratio isn't the entire story. The shell model of the nucleus explains further intricacies. The nucleons within a nucleus arrange themselves into energy levels, or shells, similar to electrons in an atom. Nuclei with "magic numbers" of protons or neutrons (2, 8, 20, 28, 50, 82, 126) exhibit exceptional stability due to completely filled shells. These "magic numbers" lead to particularly stable isotopes.
Pairing Effects
Another contributing factor to stability is the pairing effect. Pairs of protons and pairs of neutrons are more stable than unpaired nucleons. Nuclei with even numbers of both protons and neutrons (even-even nuclei) are generally more stable than those with odd numbers. Odd-odd nuclei are often the least stable.
Applications of Understanding the Band of Stability
Understanding the band of stability has significant implications across various scientific and technological fields:
Nuclear Medicine
Radioactive isotopes, many of which lie outside the band of stability, are crucial in nuclear medicine. These isotopes are used for diagnostic imaging (e.g., PET scans using isotopes like fluorine-18) and therapeutic treatments (e.g., iodine-131 for thyroid cancer). Their instability and consequent decay properties are exploited for these medical applications.
Nuclear Power
Nuclear power plants utilize the controlled nuclear fission of unstable isotopes (like uranium-235) to generate energy. The fission process results in the production of other, more stable nuclides. A deep understanding of the band of stability is crucial for managing the nuclear waste produced in this process.
Radiometric Dating
Radioactive decay processes, particularly alpha and beta decay, are fundamental to radiometric dating techniques. By measuring the ratio of parent isotopes (unstable) to daughter isotopes (stable), scientists can determine the age of geological materials and artifacts. This relies heavily on the knowledge of decay rates and half-lives of various radioactive isotopes.
Conclusion: A Dynamic and Ever-Evolving Understanding
The band of stability represents a fascinating interplay of fundamental forces, showcasing the delicate balance that determines the stability of atomic nuclei. While we have made significant strides in understanding this concept, research continues to refine our knowledge. The search for new isotopes, both stable and unstable, and further investigation into the intricacies of nuclear forces constantly expands our understanding of this crucial region in the chart of nuclides. This continued research further clarifies the behavior of matter at its most fundamental level, contributing to advancements in numerous fields, from medicine and energy production to archaeology and geology. The band of stability remains a cornerstone of nuclear science, a dynamic area that continually challenges and expands our comprehension of the universe.
Latest Posts
Related Post
Thank you for visiting our website which covers about What Is The Band Of Stability . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
