How Many Valence Electrons In F
listenit
Mar 25, 2025 · 5 min read
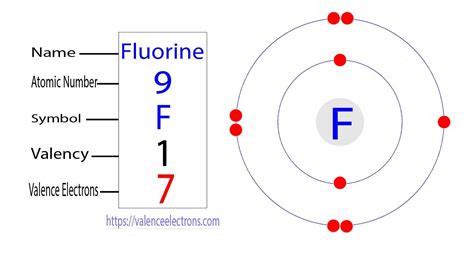
Table of Contents
How Many Valence Electrons Does Fluorine (F) Have? A Deep Dive into Atomic Structure and Chemical Behavior
Fluorine (F), the most electronegative element on the periodic table, plays a crucial role in various chemical processes. Understanding its electronic structure, specifically the number of valence electrons, is fundamental to comprehending its reactivity and bonding characteristics. This article will delve into the details of fluorine's atomic structure, explaining why it possesses seven valence electrons and how this impacts its chemical behavior.
Understanding Valence Electrons
Before focusing specifically on fluorine, let's establish a clear understanding of what valence electrons are. Valence electrons are the electrons located in the outermost shell, or energy level, of an atom. These electrons are the ones involved in chemical bonding, determining an element's reactivity and the types of bonds it can form (ionic, covalent, metallic). The number of valence electrons dictates an atom's tendency to gain, lose, or share electrons to achieve a stable electron configuration, typically resembling that of a noble gas (a full outermost shell).
Fluorine's Electronic Configuration
Fluorine's atomic number is 9, meaning it has nine protons and, in a neutral atom, nine electrons. To understand the electron arrangement, we need to consider the principle energy levels (shells) and subshells within each level. Electrons fill orbitals according to the Aufbau principle (filling lower energy levels first), Hund's rule (maximizing unpaired electrons in a subshell), and the Pauli exclusion principle (no two electrons can have the same four quantum numbers).
Fluorine's electron configuration is 1s²2s²2p⁵.
- 1s²: Two electrons occupy the 1s orbital (the lowest energy level).
- 2s²: Two electrons occupy the 2s orbital (within the second energy level).
- 2p⁵: Five electrons occupy the 2p orbitals (also within the second energy level). The 2p subshell has three orbitals (2px, 2py, 2pz), each capable of holding two electrons.
Identifying Valence Electrons in Fluorine
The valence electrons are those in the outermost shell. In fluorine's case, the outermost shell is the second energy level (n=2). This shell contains both the 2s and 2p electrons. Therefore, fluorine has a total of seven valence electrons: two from the 2s subshell and five from the 2p subshell (2 + 5 = 7).
Fluorine's Reactivity and the Octet Rule
Fluorine's seven valence electrons explain its exceptionally high reactivity. Atoms strive to achieve a stable electron configuration, often following the octet rule (having eight electrons in their outermost shell, like the noble gases). Since fluorine is only one electron short of a full octet, it has a strong tendency to gain an electron, forming a fluoride ion (F⁻). This gain of an electron results in a stable, filled outermost shell with eight electrons (1s²2s²2p⁶), achieving the noble gas configuration of neon.
This high electronegativity makes fluorine a powerful oxidizing agent, readily accepting electrons from other atoms. It reacts vigorously with most other elements, often forming ionic compounds with metals (where it gains an electron) or covalent compounds with nonmetals (where it shares electrons to achieve a stable octet).
Examples of Fluorine's Chemical Bonding
Let's examine some examples to illustrate how fluorine's seven valence electrons influence its bonding:
1. Ionic Bonding with Sodium (NaF)
Sodium (Na) has one valence electron, while fluorine has seven. Sodium readily loses its valence electron to achieve a stable octet, forming a sodium ion (Na⁺). Fluorine gains this electron, becoming a fluoride ion (F⁻). The electrostatic attraction between the oppositely charged ions forms the ionic compound sodium fluoride (NaF).
2. Covalent Bonding with Hydrogen (HF)
Hydrogen (H) has one valence electron. To achieve a stable configuration (similar to helium), it needs two electrons. Fluorine, with its seven valence electrons, can share one of its electrons with hydrogen, forming a covalent bond. This shared electron pair is attracted to both the hydrogen and fluorine nuclei, resulting in a stable molecule of hydrogen fluoride (HF). Note that even though fluorine shares an electron, it still holds the shared electrons more tightly due to its high electronegativity, leading to a polar covalent bond.
3. Covalent Bonding in Fluorine Gas (F₂)
Two fluorine atoms can form a covalent bond by sharing one electron pair. Each fluorine atom contributes one electron to the shared pair, resulting in each atom having a complete octet (eight electrons in the outermost shell). This forms the diatomic molecule fluorine gas (F₂).
Fluorine's Importance in Various Fields
The unique properties of fluorine, stemming directly from its seven valence electrons, have led to its widespread use in various fields:
- Dentistry: Fluoride ions are crucial in preventing tooth decay by strengthening tooth enamel.
- Refrigerants: Certain fluorocarbons were historically used as refrigerants, although concerns about their environmental impact have led to the development of alternative refrigerants.
- Pharmaceuticals: Fluorine is incorporated into many pharmaceuticals to alter their properties, improve their effectiveness, or enhance their stability.
- Industrial Processes: Fluorine and its compounds are employed in various industrial processes, including the production of plastics, lubricants, and other materials.
Conclusion: The Significance of Seven Valence Electrons
Fluorine's seven valence electrons are the key to understanding its remarkable chemical properties. Its strong tendency to gain an electron to achieve a stable octet leads to its high reactivity and its ability to form a wide range of compounds. The influence of these seven electrons extends across various scientific and technological fields, highlighting the importance of fundamental atomic structure in determining macroscopic properties and applications. Further research and understanding of fluorine's chemistry continue to open up new avenues of innovation and development in numerous disciplines. By grasping the essence of its electronic configuration, we can better appreciate the unique role of this element in the world around us.
Latest Posts
Latest Posts
-
What Muscle Subdivides The Ventral Body Cavity
Mar 28, 2025
-
What Are Three Main Ideas Of The Cell Theory
Mar 28, 2025
-
Hcl Ba Oh 2 Balanced Equation
Mar 28, 2025
-
7x 2y 13 X 2y 11
Mar 28, 2025
-
Is Digesting Food A Physical Or Chemical Change
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons In F . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
