Hcl Ba Oh 2 Balanced Equation
listenit
Mar 28, 2025 · 5 min read
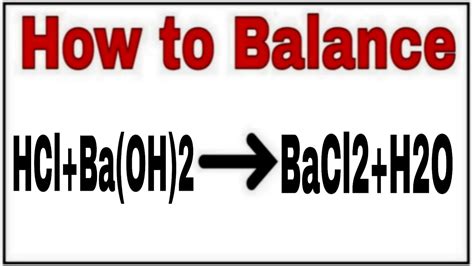
Table of Contents
Understanding the Balanced Equation for the Reaction Between HCl and Ba(OH)₂
This article delves deep into the chemical reaction between hydrochloric acid (HCl) and barium hydroxide (Ba(OH)₂), exploring the balanced equation, the stoichiometry involved, and the practical implications of this reaction. We'll also touch upon the concepts of acid-base neutralization and its applications.
The Reaction Between HCl and Ba(OH)₂: A Neutralization Reaction
Hydrochloric acid (HCl) is a strong, monoprotic acid, meaning it readily donates one proton (H⁺) per molecule. Barium hydroxide (Ba(OH)₂), on the other hand, is a strong diprotic base, capable of accepting two protons per molecule. When these two substances react, they undergo a classic acid-base neutralization reaction. This type of reaction involves the combination of an acid and a base to produce salt and water.
The initial, unbalanced equation representing this reaction is:
HCl + Ba(OH)₂ → BaCl₂ + H₂O
This equation shows the reactants (HCl and Ba(OH)₂) and the products (barium chloride (BaCl₂) and water (H₂O)). However, it's crucial to note that this equation is not balanced. A balanced equation ensures that the number of atoms of each element is equal on both the reactant and product sides, adhering to the law of conservation of mass.
Balancing the Chemical Equation
To balance the equation, we need to adjust the coefficients (the numbers in front of each chemical formula) so that the number of atoms of each element is the same on both sides. Let's break down the process:
-
Examine the Barium (Ba): There's one barium atom on each side, so barium is already balanced.
-
Examine the Chlorine (Cl): There's one chlorine atom on the reactant side (in HCl) and two on the product side (in BaCl₂). To balance chlorine, we need to place a coefficient of 2 in front of HCl:
2HCl + Ba(OH)₂ → BaCl₂ + H₂O
-
Examine the Hydrogen (H): Now we have four hydrogen atoms on the reactant side (two from 2HCl and two from Ba(OH)₂), and only two on the product side. To balance hydrogen, we need to place a coefficient of 2 in front of H₂O:
2HCl + Ba(OH)₂ → BaCl₂ + 2H₂O
-
Examine the Oxygen (O): Finally, let's check oxygen. We have two oxygen atoms on the reactant side (from Ba(OH)₂) and two on the product side (from 2H₂O). Oxygen is balanced.
Therefore, the completely balanced equation for the reaction between HCl and Ba(OH)₂ is:
2HCl + Ba(OH)₂ → BaCl₂ + 2H₂O
Stoichiometry and Mole Ratios
The balanced equation provides crucial information about the stoichiometry of the reaction, specifically the mole ratios between the reactants and products. The coefficients in the balanced equation represent the relative number of moles of each substance involved. In this case:
- 2 moles of HCl react with 1 mole of Ba(OH)₂.
- This reaction produces 1 mole of BaCl₂ and 2 moles of H₂O.
Understanding these mole ratios is essential for performing stoichiometric calculations, such as determining the amount of product formed from a given amount of reactant or vice-versa.
Types of Reactions Involved
This reaction exemplifies several important chemical reaction types:
- Neutralization Reaction: As mentioned, this is a primary classification, emphasizing the reaction between an acid and a base.
- Double Displacement Reaction (Metathesis): The reaction involves the exchange of ions between two compounds. The hydrogen ions (H⁺) from HCl combine with the hydroxide ions (OH⁻) from Ba(OH)₂ to form water, while the barium ions (Ba²⁺) and chloride ions (Cl⁻) combine to form barium chloride.
- Exothermic Reaction: This reaction releases heat to the surroundings. The formation of water is highly exothermic, contributing significantly to the overall heat released during the neutralization process.
Applications and Importance
The reaction between HCl and Ba(OH)₂ holds significant importance across various applications:
- Acid-Base Titrations: This reaction is frequently employed in titrations to determine the concentration of an unknown acid or base solution. By carefully measuring the volume of HCl required to neutralize a known volume of Ba(OH)₂, the concentration of the base can be calculated using stoichiometry.
- Chemical Synthesis: Barium chloride, a product of this reaction, is used in various applications, including in the production of other barium compounds, as a component in certain types of pigments, and as a laboratory reagent.
- Understanding Acid-Base Chemistry: Studying this reaction is fundamental to grasping the concepts of acid-base chemistry, stoichiometry, and reaction balancing. It's a cornerstone of introductory chemistry education.
- Environmental Applications: Understanding neutralization reactions is crucial in addressing environmental issues related to acid rain or industrial waste containing strong acids or bases. Neutralization processes can help mitigate the negative impacts of these substances.
Practical Considerations and Safety
When performing this reaction in a laboratory setting, several safety precautions are essential:
- Eye Protection: Always wear appropriate safety goggles to protect your eyes from splashes of chemicals.
- Gloves: Wear chemical-resistant gloves to prevent skin contact with HCl and Ba(OH)₂, which can cause irritation or burns.
- Ventilation: Conduct the experiment under a well-ventilated hood or in a well-ventilated area to minimize exposure to potentially harmful fumes.
- Careful Handling: Handle HCl and Ba(OH)₂ solutions with care, avoiding spills or contact with skin or eyes.
- Disposal: Dispose of the resulting solutions properly according to your institution's guidelines.
Further Exploration
The reaction between HCl and Ba(OH)₂ offers a rich foundation for exploring more complex chemical concepts. Further exploration might include:
- Thermochemistry: Investigating the enthalpy change (ΔH) associated with this exothermic reaction.
- Kinetics: Studying the rate of the reaction under different conditions.
- Equilibrium: Examining the equilibrium constant for the reaction if it were to be conducted in a reversible manner.
Conclusion
The balanced equation for the reaction between HCl and Ba(OH)₂, 2HCl + Ba(OH)₂ → BaCl₂ + 2H₂O, represents a fundamental acid-base neutralization reaction with significant implications in chemistry and related fields. Understanding the stoichiometry, the types of reactions involved, and the safety precautions associated with this reaction are crucial for both students and professionals working in chemistry-related fields. This reaction serves as a building block for further exploration into more advanced concepts in chemistry. By carefully examining this seemingly simple reaction, one gains valuable insights into the complexities and applications of chemical principles.
Latest Posts
Latest Posts
-
What Is The Name Of This Hydrocarbon
Mar 31, 2025
-
Columns Of Periodic Table Are Called
Mar 31, 2025
-
Difference Between Animal Mitosis And Plant Mitosis
Mar 31, 2025
-
What Is Half Of One And A Half Teaspoons
Mar 31, 2025
-
What Is 4 12 In Simplest Form
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Hcl Ba Oh 2 Balanced Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
