What Is The Smallest Unit Of Element
listenit
Mar 22, 2025 · 6 min read
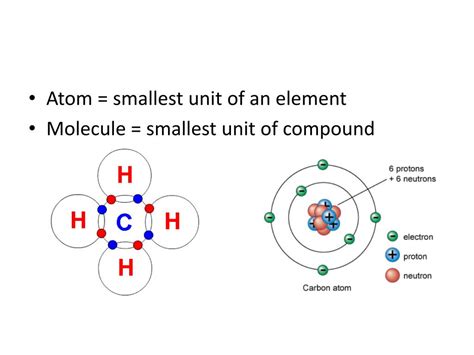
Table of Contents
What is the Smallest Unit of an Element? Delving into Atoms, Subatomic Particles, and Beyond
The question, "What is the smallest unit of an element?" seems straightforward, yet its answer unveils a fascinating journey into the heart of matter, revealing layers of complexity that continue to captivate scientists and researchers. While the simple answer is the atom, understanding the true nature of this fundamental unit requires exploring its internal structure and the even smaller particles that compose it. This article delves deep into the atomic world, exploring the historical context, the modern understanding of atomic structure, and the implications of this knowledge for various scientific fields.
The Atom: A Historical Perspective
The concept of the atom, meaning "indivisible" in Greek, dates back to ancient Greece. Philosophers like Leucippus and Democritus proposed the existence of fundamental, indivisible particles that make up all matter. However, these ideas remained largely philosophical speculations for centuries, lacking the experimental evidence needed for scientific validation.
The true scientific investigation into the atom began in the 18th and 19th centuries with advancements in chemistry. John Dalton's atomic theory (early 1800s), based on experimental observations, revived the atomic concept, proposing that elements consist of identical, indivisible atoms. This theory provided a framework for understanding chemical reactions and the law of conservation of mass.
However, Dalton's model, like its ancient predecessors, was incomplete. Subsequent discoveries demonstrated that atoms are, in fact, divisible. Experiments using cathode rays by scientists like J.J. Thomson revealed the existence of electrons, negatively charged subatomic particles. This led to the development of the "plum pudding" model, which depicted atoms as a positively charged sphere with negatively charged electrons embedded within.
The Nuclear Model and Beyond: Unveiling Subatomic Particles
Ernest Rutherford's famous gold foil experiment in 1911 revolutionized our understanding of the atom. By bombarding a thin gold foil with alpha particles, Rutherford discovered that most of the alpha particles passed straight through, while a few were deflected at large angles. This unexpected result led to the nuclear model of the atom, proposing that most of the atom's mass and all its positive charge are concentrated in a tiny, dense nucleus at the center, while electrons orbit this nucleus at a considerable distance.
This model, however, still wasn't complete. The spectral lines of elements, observed when they emit light, couldn't be explained by the simple orbiting electron model. Niels Bohr's model improved upon this by introducing the concept of quantized energy levels for electrons, suggesting that electrons only orbit the nucleus at specific energy levels. This model successfully explained the observed spectral lines.
Further investigations revealed even more complexity. The nucleus itself was found to be composed of two types of particles: protons, positively charged particles with a mass approximately 1836 times that of an electron, and neutrons, neutral particles with a mass slightly greater than that of a proton. The number of protons in an atom's nucleus determines its atomic number and identifies the element. The total number of protons and neutrons determines its mass number.
Subatomic Particles: A Deeper Dive
The discovery of protons and neutrons didn't mark the end of the story. The quest to understand the fundamental building blocks of matter continued, leading to the development of the Standard Model of particle physics. This model describes a zoo of subatomic particles, including quarks, leptons, and bosons.
Quarks: These are fundamental particles that make up protons and neutrons. There are six types, or "flavors," of quarks: up, down, charm, strange, top, and bottom. Protons consist of two up quarks and one down quark, while neutrons consist of one up quark and two down quarks.
Leptons: Electrons belong to a family of particles called leptons. Other leptons include muons and tau particles, which are heavier versions of the electron, along with their associated neutrinos.
Bosons: These are force-carrying particles that mediate interactions between other particles. The most well-known boson is the photon, which carries the electromagnetic force. Other bosons include gluons (which hold quarks together), W and Z bosons (which mediate the weak nuclear force), and the Higgs boson, responsible for giving particles mass.
Isotopes and Beyond: Variations in Atomic Structure
While the number of protons defines an element, the number of neutrons can vary. Atoms of the same element with different numbers of neutrons are called isotopes. For example, carbon-12 (⁶C) has six protons and six neutrons, while carbon-14 (¹⁴C) has six protons and eight neutrons. Isotopes have the same chemical properties but different physical properties, such as mass and radioactivity.
The concept of isotopes expands our understanding of the "smallest unit" question. While the atom is the smallest unit that retains the chemical properties of an element, different isotopes of the same element have variations in their nuclear structure.
The Implications of Atomic Structure Understanding
Our understanding of the atom and its subatomic components has profound implications across various scientific fields:
- Chemistry: Atomic structure is fundamental to understanding chemical bonding, reactivity, and the properties of different elements and compounds.
- Nuclear Physics: The study of the nucleus and its interactions leads to applications in nuclear energy, nuclear medicine, and other technologies.
- Particle Physics: The exploration of fundamental particles and forces continues to deepen our understanding of the universe's origins and evolution.
- Materials Science: Manipulating atomic structures allows for the creation of new materials with specific properties, such as superconductors and advanced semiconductors.
- Medical Imaging and Treatment: Radioactive isotopes are crucial tools in medical imaging (e.g., PET scans) and radiation therapy for cancer treatment.
The Ongoing Quest: Are There Smaller Units?
While the Standard Model of particle physics describes the fundamental particles we know, the quest to understand the smallest unit continues. Questions about dark matter and dark energy, which constitute a significant portion of the universe's mass-energy, point to the possibility of undiscovered particles and forces. String theory and other theoretical frameworks propose the existence of even smaller fundamental units than quarks and leptons.
Conclusion: A Dynamic and Evolving Understanding
Therefore, the answer to "What is the smallest unit of an element?" is nuanced. While the atom is the smallest unit that retains the chemical properties of an element, the atom itself is composed of even smaller particles, which can be further broken down into fundamental particles according to the current Standard Model. The journey from Democritus's philosophical speculations to the complex Standard Model represents a triumph of scientific inquiry. However, the exploration continues, and future discoveries may further refine our understanding of matter's ultimate building blocks, challenging our current understanding of the “smallest unit” and pushing the boundaries of scientific knowledge. The atom, while a fundamental unit, reveals a universe of complexity within its seemingly simple structure, a testament to the endless fascination of scientific exploration.
Latest Posts
Latest Posts
-
Give The Temperature And Pressure At Stp
Mar 23, 2025
-
How Many Electrons Does K Have
Mar 23, 2025
-
If The Ka Of A Monoprotic Weak Acid Is
Mar 23, 2025
-
Least Common Multiple Of 12 And 8
Mar 23, 2025
-
What Is The Percent Of 7 9
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about What Is The Smallest Unit Of Element . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
