If The Ka Of A Monoprotic Weak Acid Is
listenit
Mar 23, 2025 · 6 min read
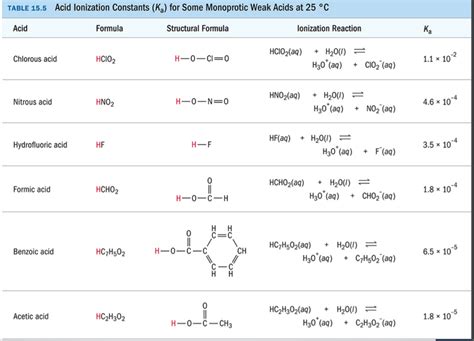
Table of Contents
If the Ka of a Monoprotic Weak Acid Is... Understanding Acid Dissociation and its Implications
The Ka value, or acid dissociation constant, is a crucial parameter in understanding the behavior of weak acids in aqueous solutions. Knowing the Ka of a monoprotic weak acid allows us to predict its degree of dissociation, the pH of its solutions, and its behavior in reactions. This article delves into the significance of Ka, exploring its calculation, implications, and applications in various contexts. We will cover a range of topics, from the fundamentals of acid-base chemistry to more advanced applications, ensuring a comprehensive understanding for both beginners and more experienced learners.
Understanding Ka: The Acid Dissociation Constant
A monoprotic weak acid is an acid that can donate only one proton (H⁺) per molecule. Unlike strong acids, which dissociate completely in water, weak acids only partially dissociate. This equilibrium is represented by the following general equation:
HA(aq) ⇌ H⁺(aq) + A⁻(aq)
Where:
- HA represents the weak acid.
- H⁺ represents the hydrogen ion (proton).
- A⁻ represents the conjugate base.
The Ka value is the equilibrium constant for this dissociation reaction and is defined as:
Ka = [H⁺][A⁻] / [HA]
Where:
- [H⁺], [A⁻], and [HA] represent the equilibrium concentrations of the hydrogen ion, conjugate base, and weak acid, respectively.
A higher Ka value indicates a stronger weak acid, meaning it dissociates more readily. Conversely, a lower Ka value signifies a weaker weak acid, with less dissociation. The pKa, which is the negative logarithm of Ka (pKa = -log₁₀Ka), is often used as a more convenient measure of acid strength. A lower pKa indicates a stronger acid.
Calculating Ka from Given Data
The Ka of a monoprotic weak acid can be determined experimentally through various methods, often involving pH measurements. Here's a common approach:
1. Titration: Titrating a weak acid with a strong base allows us to determine the concentration of the acid at various points during the titration. The pH at the half-equivalence point (where half of the acid has been neutralized) is equal to the pKa of the acid. From the pKa, the Ka can be calculated.
2. pH Measurement: Measuring the pH of a solution of known weak acid concentration allows for the calculation of Ka. The equilibrium concentrations of H⁺, A⁻, and HA can be determined from the pH, using the following steps:
- Determine [H⁺]: [H⁺] = 10⁻pH
- Determine [A⁻]: Since the dissociation is 1:1, [A⁻] = [H⁺]
- Determine [HA]: [HA] = initial concentration of HA - [H⁺]
- Calculate Ka: Substitute the determined concentrations into the Ka expression.
Example: A 0.1 M solution of a weak monoprotic acid has a pH of 3. Calculate the Ka.
- [H⁺] = 10⁻³ = 0.001 M
- [A⁻] = 0.001 M
- [HA] = 0.1 - 0.001 = 0.099 M
- Ka = (0.001)(0.001) / 0.099 ≈ 1.01 x 10⁻⁵
Implications of Ka on Acid Behavior
The Ka value significantly impacts several aspects of a weak acid's behavior:
1. Degree of Dissociation (α):
The degree of dissociation (α) represents the fraction of the acid that has dissociated. It's calculated as:
α = [H⁺] / [HA]₀
Where [HA]₀ is the initial concentration of the acid. A higher Ka leads to a higher degree of dissociation, indicating a greater extent of ionization.
2. pH of Solutions:
The pH of a weak acid solution is determined by the Ka value and the initial concentration of the acid. The pH can be calculated using the following approximation (valid when the degree of dissociation is low):
pH = 0.5(pKa - log₁₀[HA]₀)
This approximation simplifies the calculation considerably, but it is important to check its validity by comparing the calculated [H+] with the initial concentration of the acid. If [H+] is a significant fraction of [HA]₀, the quadratic formula should be used for a more accurate calculation.
3. Buffer Solutions:
Weak acids, along with their conjugate bases, form buffer solutions which resist changes in pH upon addition of small amounts of acid or base. The effectiveness of a buffer solution is maximized when the pH is close to the pKa of the weak acid. The Henderson-Hasselbalch equation describes the relationship between pH, pKa, and the concentrations of the acid and its conjugate base:
pH = pKa + log₁₀([A⁻] / [HA])
4. Acid-Base Reactions:
The Ka value determines the equilibrium position of acid-base reactions involving the weak acid. A higher Ka implies the reaction will favor product formation to a greater extent.
Advanced Applications and Considerations
1. Polyprotic Acids:
Polyprotic acids can donate more than one proton. Each dissociation step has its own Ka value (Ka1, Ka2, etc.). The Ka values for successive dissociations are typically smaller, reflecting the increasing difficulty of removing a proton from the increasingly negatively charged species.
2. Temperature Dependence:
Ka is temperature dependent. Usually, an increase in temperature increases the Ka value for weak acids, indicating a greater degree of dissociation at higher temperatures.
3. Ionic Strength Effects:
The presence of other ions in solution can affect the activity of the acid and its conjugate base, leading to deviations from the ideal behavior predicted by the simple Ka expression. Activities rather than concentrations should be used in the Ka expression for more accurate calculations in solutions of high ionic strength. The Debye-Hückel equation can be used to estimate activity coefficients.
4. Calculating Ka from Spectroscopic Data:
The Ka value of a weak acid can be determined from spectrophotometric measurements. If the acid and its conjugate base exhibit different absorption spectra, the absorbance can be used to determine the equilibrium concentrations of HA and A⁻, allowing for the calculation of Ka.
5. Ka and Drug Design:
In pharmaceutical science, the Ka values of drugs are important for determining their absorption, distribution, metabolism, and excretion (ADME) properties. Many drugs are weak acids or bases, and their ionization state influences their ability to cross cell membranes and interact with biological targets.
6. Environmental Chemistry:
Ka values are crucial in environmental chemistry for understanding the behavior of acids in natural systems. For example, the acidity of rainwater and its impact on aquatic ecosystems are partly determined by the Ka values of dissolved acids. The speciation of metals in water, a key factor in assessing metal toxicity, is heavily dependent on the pH and the Ka values of relevant metal-ligand complexes.
Conclusion: The Importance of Ka in Understanding Weak Acids
The acid dissociation constant, Ka, is a fundamental parameter for understanding and predicting the behavior of monoprotic weak acids in aqueous solutions. Its value provides essential information about the degree of dissociation, pH of solutions, buffer capacity, and the role of the weak acid in various chemical reactions. Understanding Ka's implications extends beyond basic chemistry, finding critical applications in various fields, including pharmaceutical science and environmental chemistry. Accurate determination and interpretation of Ka are key to tackling complex problems across a wide range of disciplines. By mastering the concepts and calculations related to Ka, we gain a deeper appreciation for the intricate world of acid-base chemistry and its significant impact on our world.
Latest Posts
Latest Posts
-
How To Factor X 2 9
Mar 25, 2025
-
How Many Ounces Is In A Fifth Of Alcohol
Mar 25, 2025
-
Can A Rhombus Be A Trapezoid
Mar 25, 2025
-
What Is 5 As A Percent
Mar 25, 2025
-
65 As A Fraction In Simplest Form
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about If The Ka Of A Monoprotic Weak Acid Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
