How Many Electrons Does K Have
listenit
Mar 23, 2025 · 5 min read
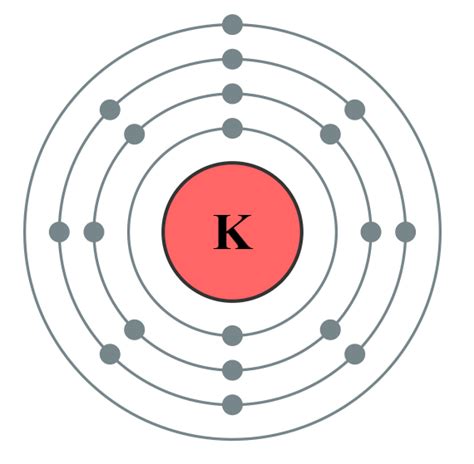
Table of Contents
How Many Electrons Does Potassium (K) Have? Understanding Atomic Structure and Electron Configuration
Potassium (K), a crucial element for human health and a key player in various industrial applications, holds a specific number of electrons that dictates its chemical behavior and properties. Understanding this number requires a dive into the fascinating world of atomic structure and electron configuration. This comprehensive guide will explore not only the answer to the question "How many electrons does K have?" but also delve into the underlying principles that govern electron arrangement within an atom.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we determine the number of electrons in potassium, let's establish a foundational understanding of atomic structure. Every atom consists of three fundamental subatomic particles:
- Protons: Positively charged particles found in the atom's nucleus. The number of protons defines the element; potassium always has 19 protons.
- Neutrons: Neutral particles (no charge) also residing in the atom's nucleus. The number of neutrons can vary within the same element, leading to isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in specific energy levels or shells. The number of electrons typically equals the number of protons in a neutral atom, ensuring overall electrical neutrality.
Determining the Number of Electrons in Potassium (K)
The atomic number of an element represents the number of protons in its nucleus. Potassium's atomic number is 19. In a neutral potassium atom, the number of electrons equals the number of protons. Therefore, a neutral potassium atom has 19 electrons.
This seemingly simple answer opens the door to a deeper exploration of how these 19 electrons are arranged within the potassium atom.
Electron Shells and Subshells: The Quantum Mechanical Model
Electrons don't orbit the nucleus randomly; they occupy specific energy levels called shells. These shells are further divided into subshells, each capable of holding a specific number of electrons. The filling of these shells and subshells follows specific rules dictated by quantum mechanics:
- Shell 1 (n=1): Contains only one subshell, the s subshell, which can hold a maximum of 2 electrons.
- Shell 2 (n=2): Contains two subshells: s (holds 2 electrons) and p (holds 6 electrons). Total capacity: 8 electrons.
- Shell 3 (n=3): Contains three subshells: s (2 electrons), p (6 electrons), and d (10 electrons). Total capacity: 18 electrons.
- Shell 4 (n=4) and beyond: The pattern continues with s, p, d, and f subshells, with increasing capacity.
Potassium's Electron Configuration: A Step-by-Step Approach
To determine the electron configuration of potassium, we systematically fill the electron shells and subshells according to the rules mentioned above:
- Shell 1: The first two electrons fill the 1s subshell (1s²).
- Shell 2: The next eight electrons fill the 2s and 2p subshells (2s²2p⁶).
- Shell 3: The next eight electrons fill the 3s and 3p subshells (3s²3p⁶).
- Shell 4: The remaining electron occupies the 4s subshell (4s¹).
Therefore, the complete electron configuration of potassium is 1s²2s²2p⁶3s²3p⁶4s¹. This configuration clearly demonstrates that potassium possesses 19 electrons, distributed across four shells.
The Significance of the Outermost Electron: Valence Electrons and Chemical Reactivity
The outermost shell of an atom is called the valence shell, and the electrons in this shell are known as valence electrons. These electrons play a crucial role in determining an element's chemical reactivity. Potassium, with its single valence electron in the 4s subshell, is highly reactive. It readily loses this electron to achieve a stable electron configuration resembling that of the noble gas argon (1s²2s²2p⁶3s²3p⁶). This tendency to lose an electron makes potassium a highly electropositive element.
Isotopes of Potassium: Variations in Neutron Number
While the number of electrons in a neutral potassium atom remains consistently 19, the number of neutrons can vary, resulting in different isotopes. Isotopes are atoms of the same element with the same number of protons but differing numbers of neutrons. The most common isotopes of potassium are:
- Potassium-39 (³⁹K): This is the most abundant isotope, comprising about 93.3% of natural potassium. It contains 19 protons and 20 neutrons.
- Potassium-40 (⁴⁰K): A radioactive isotope making up about 0.012% of natural potassium. It contains 19 protons and 21 neutrons. This isotope undergoes radioactive decay, emitting beta particles and gamma rays.
- Potassium-41 (⁴¹K): Another stable isotope, making up around 6.7% of natural potassium. It contains 19 protons and 22 neutrons.
Although isotopes have different numbers of neutrons, the number of electrons in a neutral atom of any potassium isotope remains 19. The difference in neutron number impacts the atom's mass but not its chemical behavior.
Potassium's Importance in Biology and Industry
Potassium's unique electron configuration and resulting chemical properties contribute significantly to its importance across various fields:
- Biological Importance: Potassium ions (K⁺) are essential for numerous biological processes, including maintaining fluid balance, nerve impulse transmission, muscle contraction, and enzyme activity. Its role in maintaining the resting membrane potential of cells is critical for cellular function.
- Industrial Applications: Potassium is used in various industrial processes, including the production of fertilizers (potassium is a crucial macronutrient for plant growth), soaps, and glass. Potassium compounds are also employed in pharmaceuticals and specialized chemicals.
Conclusion: Understanding the Bigger Picture
The seemingly simple question "How many electrons does K have?" opens a gateway to understanding the complex world of atomic structure, electron configuration, and chemical reactivity. The 19 electrons in potassium, distributed according to its unique electron configuration, determine its chemical behavior, its biological significance, and its various industrial applications. This understanding highlights the fundamental link between an element's atomic structure and its macroscopic properties and roles in the world around us. Furthermore, exploring the concepts of isotopes reinforces the diversity within even a single element and contributes to a more comprehensive understanding of its behavior and applications. The exploration extends beyond simply stating the number of electrons, fostering deeper scientific literacy and appreciation for the intricate world of chemistry.
Latest Posts
Latest Posts
-
Common Factors Of 45 And 75
Mar 25, 2025
-
6 Out Of 11 As A Percentage
Mar 25, 2025
-
How Do Compounds Differ From Elements
Mar 25, 2025
-
What Percent Is 7 Out Of 20
Mar 25, 2025
-
Which Color Of Light Has The Most Energy
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Does K Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
