How Do Compounds Differ From Elements
listenit
Mar 25, 2025 · 7 min read
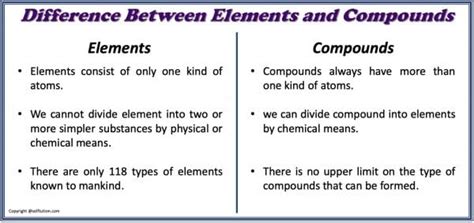
Table of Contents
How Do Compounds Differ From Elements? A Deep Dive into Chemical Structure and Properties
Understanding the fundamental building blocks of matter is crucial to grasping the complexities of chemistry. At the heart of this understanding lies the distinction between elements and compounds. While seemingly simple, the differences between these two categories are profound and impact nearly every aspect of the physical world around us. This article will delve into the core differences between elements and compounds, exploring their composition, properties, and the chemical processes that govern their interactions.
Defining Elements: The Fundamental Building Blocks
Elements are the simplest form of matter that cannot be broken down into simpler substances by chemical means. Each element is defined by its unique atomic number, which represents the number of protons in the nucleus of its atom. This number dictates the element's identity and its position on the periodic table. The periodic table itself is a powerful tool that organizes elements based on their atomic structure and recurring chemical properties.
Key Characteristics of Elements:
- Pure Substances: Elements consist of only one type of atom. This means all atoms within a sample of an element have the same number of protons.
- Unique Properties: Each element possesses a unique set of physical and chemical properties, including melting point, boiling point, density, reactivity, and conductivity. These properties arise directly from the element's atomic structure, specifically the arrangement of electrons.
- Cannot be Broken Down: Elements cannot be further simplified by chemical reactions. While nuclear reactions can transform elements, this is a different process entirely and lies outside the realm of traditional chemistry.
- Represented by Symbols: Elements are concisely represented by chemical symbols, usually one or two letters derived from their names (e.g., H for hydrogen, O for oxygen, Fe for iron).
Defining Compounds: Combining Elements into New Substances
Compounds are substances formed when two or more different elements are chemically bonded together in fixed proportions. This bonding involves the sharing or transfer of electrons between atoms, resulting in a new substance with properties distinct from its constituent elements. The combination is not a simple mixture, but rather a fundamental change in the chemical nature of the elements involved.
Key Characteristics of Compounds:
- Fixed Composition: Compounds always contain the same elements in the same proportions by mass. This is expressed by their chemical formula, which indicates the type and number of atoms present in a single unit of the compound (e.g., H₂O for water, NaCl for table salt).
- Unique Properties: The properties of a compound are generally very different from the properties of the elements that constitute it. For instance, sodium (Na) is a highly reactive metal and chlorine (Cl) is a toxic gas, yet their combination, sodium chloride (NaCl), is the familiar, non-toxic table salt.
- Can be Broken Down: Compounds can be decomposed into their constituent elements through chemical reactions, such as electrolysis or thermal decomposition. This decomposition involves breaking the chemical bonds holding the atoms together.
- Represented by Formulas: Compounds are represented by chemical formulas, which show the types and numbers of atoms present in a molecule or formula unit of the compound.
The Crucial Differences Between Elements and Compounds: A Comparative Analysis
The differences between elements and compounds are best understood through a direct comparison:
| Feature | Element | Compound |
|---|---|---|
| Composition | One type of atom | Two or more different types of atoms |
| Bonding | No chemical bonds between atoms | Chemical bonds (ionic, covalent, metallic) |
| Properties | Unique properties | Properties different from constituent elements |
| Decomposition | Cannot be broken down chemically | Can be broken down chemically into elements |
| Representation | Chemical symbol (e.g., H, O, Fe) | Chemical formula (e.g., H₂O, NaCl, CO₂) |
| Examples | Hydrogen (H), Oxygen (O), Iron (Fe) | Water (H₂O), Salt (NaCl), Carbon Dioxide (CO₂) |
Types of Chemical Bonds in Compounds
The formation of a compound relies heavily on the type of chemical bond formed between the constituent elements. Several key types exist:
1. Ionic Bonds: The Transfer of Electrons
Ionic bonds are formed through the electrostatic attraction between oppositely charged ions. This occurs when one atom donates one or more electrons to another atom, creating a positively charged cation and a negatively charged anion. These ions are then held together by the strong electrostatic forces between them. Ionic compounds typically form between metals and nonmetals. Examples include sodium chloride (NaCl) and magnesium oxide (MgO).
2. Covalent Bonds: The Sharing of Electrons
Covalent bonds involve the sharing of one or more pairs of electrons between two atoms. This sharing creates a stable molecular structure where the atoms are held together by the attractive forces between the shared electrons and the positively charged nuclei. Covalent compounds typically form between nonmetals. Examples include water (H₂O), methane (CH₄), and carbon dioxide (CO₂).
3. Metallic Bonds: A Sea of Electrons
Metallic bonds occur in metals, where valence electrons are delocalized and form a "sea" of electrons that surrounds the positively charged metal ions. This sea of electrons allows for the high electrical and thermal conductivity characteristic of metals.
Exploring the Properties of Compounds: A Deeper Look
The properties of a compound are significantly influenced by the types of elements present and the nature of the chemical bonds holding them together. Several key properties are worth exploring:
1. Physical Properties:
- Melting and Boiling Points: These depend on the strength of the intermolecular forces between the molecules or ions in the compound. Stronger forces lead to higher melting and boiling points.
- Solubility: The ability of a compound to dissolve in a solvent (like water) depends on the polarity of the compound and the solvent. Polar compounds tend to dissolve in polar solvents, while nonpolar compounds dissolve in nonpolar solvents.
- Density: The mass per unit volume of a compound is determined by the arrangement of atoms and the mass of the constituent elements.
- Color: The color of a compound arises from the absorption and reflection of light by the electrons in the compound's molecules or ions.
2. Chemical Properties:
- Reactivity: The tendency of a compound to undergo chemical reactions depends on the types of elements present and the strength of the chemical bonds.
- Acidity/Basicity: The pH of a compound reflects its ability to donate or accept protons (H⁺ ions). Acids donate protons, while bases accept protons.
- Combustion: The ability of a compound to burn in the presence of oxygen, often releasing heat and light.
The Importance of Understanding the Difference: Applications and Implications
The distinction between elements and compounds is fundamental to numerous fields:
- Material Science: Understanding the properties of compounds allows for the design and synthesis of new materials with specific properties, such as strength, conductivity, or reactivity.
- Medicine: The properties of compounds determine their therapeutic effects and potential toxicity. Drug discovery and development heavily rely on understanding chemical structures and their interactions with biological systems.
- Environmental Science: Understanding the chemical composition and properties of compounds is crucial for monitoring pollution, assessing environmental risks, and developing remediation strategies.
- Food Science: The chemical composition of food influences its nutritional value, taste, texture, and shelf life.
- Industrial Chemistry: The production of various chemicals and materials relies on understanding chemical reactions and the properties of compounds.
Conclusion: A Foundation for Chemical Understanding
The distinction between elements and compounds is a cornerstone of chemical understanding. Elements, the fundamental building blocks, combine to form an incredibly diverse array of compounds with unique properties. The nature of the chemical bonds, the types of elements involved, and the resulting properties shape our world and drive countless technological advancements. By appreciating these fundamental differences, we unlock the potential to understand and manipulate matter for the benefit of humanity. Further exploration into the specific properties of individual elements and compounds will reveal the vast complexities and intricacies of the chemical world, revealing new possibilities for discovery and innovation.
Latest Posts
Latest Posts
-
What Is The Least Common Multiple Of 14 And 21
Mar 26, 2025
-
What Is 10 Minutes In Hours
Mar 26, 2025
-
What Is The Greatest Common Factor Of 12 And 15
Mar 26, 2025
-
What Is Oxidized And Reduced In Cellular Respiration
Mar 26, 2025
-
Chemical Reaction Of Iron And Oxygen
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about How Do Compounds Differ From Elements . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
