What Is Oxidized And Reduced In Cellular Respiration
listenit
Mar 26, 2025 · 7 min read
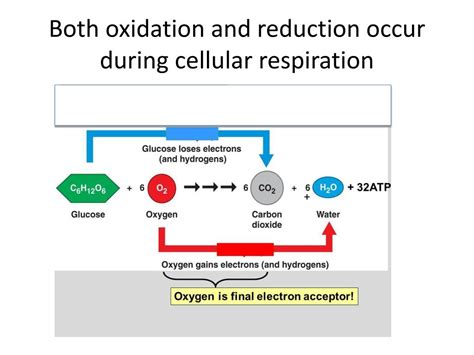
Table of Contents
What is Oxidized and Reduced in Cellular Respiration?
Cellular respiration, the process by which cells break down glucose to generate energy in the form of ATP (adenosine triphosphate), is a complex series of redox reactions. Understanding which molecules are oxidized and reduced is crucial to grasping the entire process. This article will delve deep into the oxidation and reduction reactions occurring in each stage of cellular respiration: glycolysis, pyruvate oxidation, the Krebs cycle (citric acid cycle), and oxidative phosphorylation.
The Fundamentals of Redox Reactions
Before diving into the intricacies of cellular respiration, let's review the basic principles of redox reactions. Redox, short for reduction-oxidation, refers to chemical reactions involving the transfer of electrons.
-
Oxidation: Oxidation is the loss of electrons by a molecule or atom. It often involves an increase in oxidation state (a measure of the degree of oxidation of an atom). Think of it as something "giving away" electrons.
-
Reduction: Reduction is the gain of electrons by a molecule or atom. It involves a decrease in oxidation state. Think of it as something "accepting" electrons.
These reactions always occur together; you can't have oxidation without reduction, and vice versa. The molecule that loses electrons is called the reducing agent (it causes the reduction of another molecule), while the molecule that gains electrons is called the oxidizing agent (it causes the oxidation of another molecule).
Glycolysis: The Initial Steps
Glycolysis, the first stage of cellular respiration, takes place in the cytoplasm and doesn't require oxygen. It involves a series of ten enzyme-catalyzed reactions that break down one molecule of glucose (C₆H₁₂O₆) into two molecules of pyruvate (C₃H₄O₃).
Oxidation in Glycolysis:
The key oxidation step in glycolysis occurs when glyceraldehyde-3-phosphate (G3P) is oxidized. Two molecules of G3P are produced from each glucose molecule. During this oxidation, two electrons and a proton (H⁺) are transferred from G3P to the coenzyme NAD⁺ (nicotinamide adenine dinucleotide), reducing it to NADH. This NADH carries the high-energy electrons to the electron transport chain later in cellular respiration. The oxidized G3P then undergoes further reactions to form pyruvate.
In essence: G3P is oxidized (loses electrons), and NAD⁺ is reduced (gains electrons).
Reduction in Glycolysis:
While the primary focus is on the oxidation of G3P, a small reduction step also takes place during glycolysis. The molecule 1,3-bisphosphoglycerate is reduced by the addition of a phosphate group, ultimately contributing to ATP production through substrate-level phosphorylation. Although this involves a phosphate group transfer and not a direct electron transfer, it can be considered a form of reduction in the broader context of redox reactions within the metabolic pathway.
Pyruvate Oxidation: Transition to the Mitochondria
After glycolysis, the two pyruvate molecules enter the mitochondria, the powerhouse of the cell. Here, pyruvate oxidation occurs, preparing pyruvate for entry into the Krebs cycle.
Oxidation in Pyruvate Oxidation:
In this preparatory step, each pyruvate molecule undergoes a series of reactions. Crucially, it is oxidized. One carbon atom is removed from pyruvate as carbon dioxide (CO₂), a waste product. The remaining two-carbon molecule, an acetyl group, is then attached to coenzyme A (CoA), forming acetyl-CoA. Simultaneously, two electrons and a proton are transferred to NAD⁺, reducing it to NADH.
In essence: Pyruvate is oxidized (loses electrons and carbon), and NAD⁺ is reduced (gains electrons).
The Krebs Cycle: Extracting More Energy
The Krebs cycle, also known as the citric acid cycle, is a cyclical series of reactions that further oxidizes the acetyl group derived from pyruvate. It occurs in the mitochondrial matrix.
Oxidation in the Krebs Cycle:
Multiple oxidation reactions take place within the Krebs cycle. The acetyl group is completely oxidized, releasing all its carbon atoms as CO₂. In the process, electrons are harvested and transferred to the electron carriers NAD⁺ and FAD (flavin adenine dinucleotide).
- Oxidation of acetyl-CoA: When acetyl-CoA enters the cycle, its acetyl group is oxidized.
- Oxidation of isocitrate: Isocitrate is oxidized to α-ketoglutarate, releasing CO₂ and reducing NAD⁺ to NADH.
- Oxidation of α-ketoglutarate: α-ketoglutarate is oxidized to succinyl-CoA, releasing CO₂ and reducing NAD⁺ to NADH.
- Oxidation of succinate: Succinate is oxidized to fumarate, reducing FAD to FADH₂.
- Oxidation of malate: Malate is oxidized to oxaloacetate, reducing NAD⁺ to NADH.
In essence: Multiple intermediates within the Krebs cycle are oxidized (lose electrons and carbons), reducing NAD⁺ to NADH and FAD to FADH₂.
Reduction in the Krebs Cycle:
The reduction reactions in the Krebs cycle are directly coupled with the oxidation reactions. As mentioned above, NAD⁺ is reduced to NADH and FAD is reduced to FADH₂. These reduced coenzymes carry high-energy electrons to the electron transport chain.
Oxidative Phosphorylation: The Electron Transport Chain and Chemiosmosis
Oxidative phosphorylation is the final stage of cellular respiration, and it’s where the majority of ATP is produced. It occurs in the inner mitochondrial membrane. This stage involves two main components: the electron transport chain and chemiosmosis.
Oxidation in the Electron Transport Chain:
The electron transport chain is a series of protein complexes embedded in the inner mitochondrial membrane. The electrons carried by NADH and FADH₂ are passed along this chain. As electrons move down the chain, they lose energy, which is used to pump protons (H⁺) from the mitochondrial matrix into the intermembrane space, creating a proton gradient. The final electron acceptor is oxygen (O₂), which is reduced to form water (H₂O).
In essence: NADH and FADH₂ are oxidized (lose electrons), and oxygen is reduced (gains electrons).
Reduction in Chemiosmosis:
The proton gradient generated by the electron transport chain creates a proton motive force. This force drives protons back into the mitochondrial matrix through ATP synthase, an enzyme that synthesizes ATP from ADP and inorganic phosphate (Pi). This process is called chemiosmosis. While not a direct electron transfer, the movement of protons is a key part of the overall redox process. The energy released from the movement of protons allows for the phosphorylation of ADP to ATP. The oxygen ultimately acts as the final electron acceptor in the redox chain, resulting in the formation of water and effectively allowing the chain to continually accept electrons.
Summary of Oxidation and Reduction in Cellular Respiration
To summarize, the following molecules are oxidized and reduced during cellular respiration:
| Stage | Oxidized Molecule(s) | Reduced Molecule(s) |
|---|---|---|
| Glycolysis | Glyceraldehyde-3-phosphate (G3P) | NAD⁺ |
| Pyruvate Oxidation | Pyruvate | NAD⁺ |
| Krebs Cycle | Acetyl-CoA, Isocitrate, α-ketoglutarate, Succinate, Malate | NAD⁺, FAD |
| Oxidative Phosphorylation | NADH, FADH₂ | O₂ (Oxygen) |
This table highlights the key redox reactions that drive the energy production in cellular respiration. The sequential transfer of electrons from organic molecules to electron carriers and ultimately to oxygen allows for the efficient generation of ATP, the primary energy currency of cells. Understanding these redox reactions is key to understanding how cells harness energy from glucose.
Beyond the Basics: Fine-tuning and Regulation
The descriptions above provide a simplified overview of the oxidation and reduction events in cellular respiration. In reality, the process is far more intricate, involving numerous intermediate steps and regulatory mechanisms. Enzyme activity is tightly controlled, ensuring the efficient and coordinated flow of metabolites through the pathway. The cellular environment influences the rate of respiration, and imbalances can lead to metabolic disorders. Further research into the specific enzyme mechanisms and regulatory pathways provides a more complete understanding of cellular respiration's complex dynamics. Furthermore, the efficiency of the process can vary based on factors such as temperature, pH, and the availability of substrates. These factors influence the activity of enzymes involved in the different stages and, therefore, affect the rate of ATP production. Investigating these aspects reveals the remarkable precision and adaptability of cellular respiration.
Conclusion
Cellular respiration is a marvel of biological engineering, a testament to the elegance and efficiency of biochemical processes. The intricate interplay of oxidation and reduction reactions ensures the efficient extraction of energy from glucose, powering the countless activities of living cells. By understanding the specifics of which molecules are oxidized and reduced at each stage, we gain a deeper appreciation for the fundamental principles that govern life itself. The continued study of this process will undoubtedly reveal even more fascinating details about its regulation and potential for therapeutic interventions.
Latest Posts
Latest Posts
-
What Does Ate Mean In Chemistry
Mar 29, 2025
-
Standard Enthalpy Of Formation Of Ethanol
Mar 29, 2025
-
Do Parallelograms Have 4 Right Angles
Mar 29, 2025
-
Least Common Multiple Of 10 And 8
Mar 29, 2025
-
Name 3 Ways To Dissolve Something Faster
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Is Oxidized And Reduced In Cellular Respiration . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
