Give The Temperature And Pressure At Stp
listenit
Mar 23, 2025 · 6 min read
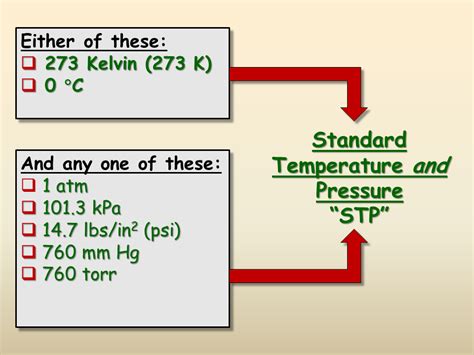
Table of Contents
Give the Temperature and Pressure at STP: A Comprehensive Guide
Standard Temperature and Pressure (STP) are reference points used in scientific calculations, particularly in chemistry and physics, to standardize the conditions under which experiments and measurements are conducted. Understanding STP is crucial for accurate comparisons and interpretations of experimental data. This comprehensive guide will delve deep into the definition of STP, its historical evolution, its applications, and common misconceptions surrounding it.
What is Standard Temperature and Pressure (STP)?
STP defines a specific temperature and pressure for a gas or substance. These values provide a baseline for comparing the properties of substances under standardized conditions, eliminating the confounding effects of varying environmental factors. While the precise definition of STP has evolved over time, the most commonly accepted values currently are:
- Temperature: 273.15 Kelvin (K), equivalent to 0 degrees Celsius (°C) or 32 degrees Fahrenheit (°F).
- Pressure: 1 atmosphere (atm), equivalent to 101.325 kilopascals (kPa), 760 millimeters of mercury (mmHg), or 14.696 pounds per square inch (psi).
It's vital to remember that these values represent standard conditions, and actual environmental conditions can and will deviate significantly.
The Evolution of STP Definitions: A Historical Perspective
The concept of STP isn't static. The values assigned to standard temperature and pressure have been refined throughout history, reflecting advancements in measurement technology and scientific understanding.
Historically, various organizations and scientific communities used slightly differing STP definitions. This variation often led to inconsistencies and difficulties in comparing experimental results across different studies. The lack of a universally accepted standard hindered collaborative efforts and the accurate interpretation of scientific findings.
The adoption of a more consistent definition of STP aimed to address this issue, facilitating broader scientific collaboration and ensuring more reliable data comparisons across different laboratories and research institutions. This transition highlighted the importance of standardization in science, emphasizing that standardized conditions are essential for generating reproducible and valid experimental results.
The current definition, while widely accepted, still might encounter situations where alternative standards are more appropriate. The choice of STP or alternative standard conditions often depends on the specific scientific context and the nature of the substances being studied. The key is consistency within a given study or experiment.
Applications of STP in Science and Engineering
The applications of STP are extensive and span various scientific disciplines and engineering fields. Some key areas include:
1. Gas Law Calculations:
STP is frequently used in calculations involving gas laws such as the Ideal Gas Law (PV=nRT). The standard conditions simplify these calculations by eliminating the need to account for varying temperature and pressure, allowing for a more straightforward determination of properties such as gas volume, molar mass, or number of moles.
2. Chemical Reactions:
In chemistry, STP is often utilized to standardize reaction conditions. By conducting reactions under STP, researchers can accurately compare reaction rates, yields, and other parameters, ensuring the reproducibility and reliability of experimental results. This is crucial in various chemical applications, from synthesizing new compounds to analyzing reaction mechanisms.
3. Thermodynamic Calculations:
STP plays a significant role in thermodynamic calculations, providing a reference point for comparing the thermodynamic properties of substances under standardized conditions. This is particularly important in fields such as materials science and chemical engineering, where understanding the thermodynamic behavior of substances is crucial for design and optimization.
4. Environmental Science:
In environmental science, STP is often used as a reference point when analyzing gas concentrations in the atmosphere or other environmental samples. Standardizing the conditions facilitates accurate comparisons of pollutant levels across different locations and time periods, assisting in environmental monitoring and pollution control efforts.
5. Engineering Applications:
STP finds applications in various engineering disciplines. For example, in mechanical engineering, it aids in the design and analysis of systems involving gases, such as combustion engines or pneumatic systems. In chemical engineering, it is integral in the design and operation of chemical processes involving gases or volatile substances.
Common Misconceptions about STP
Several common misconceptions surround STP. Understanding these misconceptions is crucial for accurately applying STP in scientific calculations and interpretations.
1. STP is universally applicable: While widely used, STP might not be the most suitable standard for all applications. For example, certain gases might exhibit non-ideal behavior at STP, requiring alternative standard conditions for accurate modeling.
2. STP is the only standard: Other standard conditions exist, particularly NTP (Normal Temperature and Pressure), which uses slightly different values (20 °C and 1 atm). The choice of standard depends on the specific context and application.
3. STP accounts for all variables: STP standardizes only temperature and pressure. Other variables, such as humidity and the presence of other gases, still affect the properties of substances and must be considered independently.
4. STP is a fixed constant: While the commonly accepted values are relatively constant, minor variations in STP definitions exist across different organizations and scientific communities. Always clarify which definition is being used to avoid ambiguity.
Beyond STP: Other Standard Conditions
While STP is widely employed, several other standard conditions are utilized depending on the specific scientific context:
-
Normal Temperature and Pressure (NTP): Typically defined as 20°C (293.15 K) and 1 atm (101.325 kPa). NTP is often preferred in certain applications, particularly those involving atmospheric gases or conditions closer to ambient temperatures.
-
Standard Ambient Temperature and Pressure (SATP): Defined as 25°C (298.15 K) and 1 atm (101.325 kPa). SATP is frequently used in some areas of chemistry and is closer to typical laboratory conditions.
-
Standard Cubic Feet (SCF): This unit corrects gas volumes to a standard temperature and pressure, usually 60°F (15.6°C) and 14.7 psi (101.325 kPa). This is common in the oil and gas industry.
The selection of appropriate standard conditions necessitates a thorough consideration of the particular application and the behavior of the substances under investigation.
Importance of Consistent Units and Reporting
Maintaining consistency in units is paramount when working with STP or any standard condition. Errors can easily arise if different units are mixed within a calculation, leading to inaccurate results. Always ensure that all units are consistent within the calculation and report units clearly in any results or findings.
Furthermore, clearly reporting the standard conditions used is crucial for reproducibility. When publishing scientific work, explicitly stating the standard conditions (whether STP, NTP, SATP, or other) enables others to replicate the experiment and validate the findings. This transparent reporting contributes to the integrity and reliability of scientific research.
Conclusion: The Continuing Relevance of STP
Standard Temperature and Pressure (STP) remains a critical concept in various scientific disciplines and engineering fields. Understanding its definition, historical evolution, applications, and limitations is crucial for accurate scientific calculations, reliable data interpretation, and effective communication within the scientific community. While other standard conditions might be more appropriate in certain scenarios, the widespread acceptance and application of STP underscore its continuing relevance in scientific research and technological advancements. The key takeaway is the importance of clarity, consistency, and appropriate selection of standard conditions for ensuring the reliability and reproducibility of scientific experiments and engineering applications.
Latest Posts
Latest Posts
-
What Percent Is 7 Out Of 20
Mar 25, 2025
-
Which Color Of Light Has The Most Energy
Mar 25, 2025
-
Differentiate Between Empirical Formula And Molecular Formula
Mar 25, 2025
-
What Is 1 7 8 As A Decimal
Mar 25, 2025
-
What Is The Square Root 125
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Give The Temperature And Pressure At Stp . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
