Differentiate Between Empirical Formula And Molecular Formula
listenit
Mar 25, 2025 · 6 min read
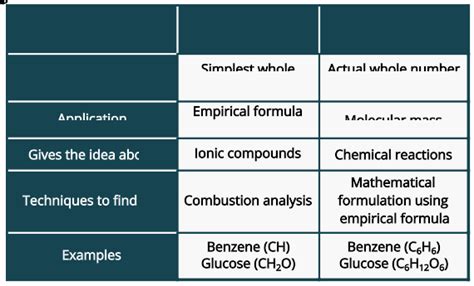
Table of Contents
- Differentiate Between Empirical Formula And Molecular Formula
- Table of Contents
- Differentiating Between Empirical Formula and Molecular Formula: A Comprehensive Guide
- What is an Empirical Formula?
- Key Characteristics of Empirical Formulas:
- Examples of Empirical Formulas:
- What is a Molecular Formula?
- Key Characteristics of Molecular Formulas:
- Examples of Molecular Formulas:
- Determining Empirical and Molecular Formulas: A Step-by-Step Guide
- Determining the Empirical Formula:
- Determining the Molecular Formula:
- Differences Summarized:
- Real-World Applications:
- Conclusion:
- Latest Posts
- Latest Posts
- Related Post
Differentiating Between Empirical Formula and Molecular Formula: A Comprehensive Guide
Understanding the difference between empirical and molecular formulas is crucial for anyone studying chemistry. While both represent the composition of a compound, they provide different levels of information. This comprehensive guide will delve into the nuances of each, explaining their definitions, applications, and how to determine one from the other. We'll also explore real-world examples to solidify your understanding.
What is an Empirical Formula?
An empirical formula represents the simplest whole-number ratio of atoms of each element present in a compound. It doesn't necessarily reflect the actual number of atoms in a molecule; rather, it shows the ratio of elements in their simplest form. Think of it as the most reduced form of a compound's elemental composition. For instance, the empirical formula doesn't tell you how many atoms are in the molecule, only the ratio between them.
Key Characteristics of Empirical Formulas:
- Simplest Ratio: Always expresses the ratio of elements in the smallest whole numbers.
- Doesn't Show Arrangement: Provides no information about the arrangement of atoms within the molecule.
- Multiple Compounds: Several different compounds can share the same empirical formula.
- Derived from Experimental Data: Primarily determined through experimental analysis, such as combustion analysis or elemental analysis.
Examples of Empirical Formulas:
- Water (H₂O): The empirical formula is also H₂O. The ratio of hydrogen to oxygen is already in its simplest form (2:1).
- Hydrogen Peroxide (H₂O₂): The empirical formula is HO. The ratio 2:2 simplifies to 1:1.
- Glucose (C₆H₁₂O₆): The empirical formula is CH₂O. The ratio 6:12:6 simplifies to 1:2:1.
What is a Molecular Formula?
A molecular formula provides the actual number of atoms of each element present in one molecule of a compound. It gives a complete representation of the molecule's composition, showing the exact number of each type of atom. This information is crucial for understanding a molecule's properties and its behavior in chemical reactions.
Key Characteristics of Molecular Formulas:
- Actual Number of Atoms: Shows the precise number of each atom type in a single molecule.
- Represents the Actual Molecule: Gives a true representation of the molecule's composition.
- Unique to Each Compound: A unique molecular formula exists for each distinct compound.
- Often a Multiple of the Empirical Formula: The molecular formula is often a whole-number multiple of the empirical formula.
Examples of Molecular Formulas:
- Water (H₂O): The molecular formula is H₂O. This directly represents one molecule of water.
- Hydrogen Peroxide (H₂O₂): The molecular formula is H₂O₂, accurately depicting two hydrogen atoms and two oxygen atoms per molecule.
- Glucose (C₆H₁₂O₆): The molecular formula is C₆H₁₂O₆, showing six carbon, twelve hydrogen, and six oxygen atoms per molecule.
Determining Empirical and Molecular Formulas: A Step-by-Step Guide
Let's explore how to determine both empirical and molecular formulas using practical examples and calculations.
Determining the Empirical Formula:
The process typically involves these steps:
-
Determine the mass of each element: This can be obtained from experimental data, such as combustion analysis or elemental analysis. The data might be given as percentages by mass or as actual masses.
-
Convert mass to moles: Divide the mass of each element by its molar mass (atomic weight) to find the number of moles of each element.
-
Find the mole ratio: Divide the number of moles of each element by the smallest number of moles calculated in step 2. This gives the simplest whole-number ratio of atoms.
-
Express the empirical formula: Write the empirical formula using the whole-number mole ratios obtained in step 3 as subscripts.
Example:
A compound is found to contain 75% carbon and 25% hydrogen by mass. Determine its empirical formula.
-
Assume 100g of the compound: This simplifies the calculations. We have 75g of carbon and 25g of hydrogen.
-
Convert to moles:
- Moles of carbon = 75g / 12.01 g/mol ≈ 6.24 mol
- Moles of hydrogen = 25g / 1.01 g/mol ≈ 24.75 mol
-
Find the mole ratio: Divide both mole values by the smaller value (6.24 mol):
- Carbon: 6.24 mol / 6.24 mol = 1
- Hydrogen: 24.75 mol / 6.24 mol ≈ 4
-
Empirical Formula: The empirical formula is CH₄ (methane).
Determining the Molecular Formula:
Once the empirical formula is known, the molecular formula can be determined if the molar mass of the compound is also known. This is achieved through the following steps:
-
Calculate the empirical formula mass: Add the atomic masses of the atoms in the empirical formula.
-
Find the ratio of molar mass to empirical formula mass: Divide the molar mass of the compound (obtained experimentally) by the empirical formula mass. This gives a whole-number multiple (n).
-
Multiply the subscripts in the empirical formula by 'n': Multiply the subscripts of each element in the empirical formula by the whole-number multiple (n) obtained in step 2. This yields the molecular formula.
Example:
The empirical formula of a compound is CH₂O, and its molar mass is determined experimentally to be 180 g/mol. Determine the molecular formula.
-
Empirical formula mass: (12.01 g/mol) + (2 × 1.01 g/mol) + (16.00 g/mol) = 30.03 g/mol
-
Ratio of molar mass to empirical formula mass: 180 g/mol / 30.03 g/mol ≈ 6
-
Molecular Formula: Multiply the subscripts in CH₂O by 6: C₆H₁₂O₆ (glucose).
Differences Summarized:
| Feature | Empirical Formula | Molecular Formula |
|---|---|---|
| Definition | Simplest whole-number ratio of atoms | Actual number of atoms in a molecule |
| Information | Ratio of elements only | Exact number and type of atoms |
| Uniqueness | Multiple compounds may have the same formula | Unique to each compound |
| Determination | From experimental mass data | Requires empirical formula and molar mass |
| Representation | Simplest form | True composition of the molecule |
Real-World Applications:
Understanding the difference between empirical and molecular formulas is essential in various fields:
- Chemistry Research: Determining the composition of newly synthesized compounds and analyzing reaction products.
- Material Science: Characterizing the composition of materials for specific applications.
- Biochemistry: Analyzing the composition of biomolecules like proteins and carbohydrates.
- Forensic Science: Identifying unknown substances found at crime scenes.
- Environmental Science: Analyzing the composition of pollutants and contaminants.
Conclusion:
The distinction between empirical and molecular formulas is fundamental to understanding chemical composition. While the empirical formula provides a simplified representation of the elemental ratio, the molecular formula offers a complete depiction of the molecule. Mastering the ability to determine both is a crucial skill for any aspiring chemist or scientist working with chemical compounds. By following the step-by-step guides provided, you can confidently navigate the world of chemical formulas and their applications. Remember to always consider the context and the information available when deciding which type of formula is most appropriate for a given situation.
Latest Posts
Latest Posts
-
Quotations Before Or After The Period
Mar 26, 2025
-
How Many Gallons Are In 12 Pints
Mar 26, 2025
-
Why Do Atoms Lose Or Gain Electrons
Mar 26, 2025
-
What Does Low Specific Heat Mean
Mar 26, 2025
-
How Many Electrons Are In This Atom
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Differentiate Between Empirical Formula And Molecular Formula . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
