Why Do Atoms Lose Or Gain Electrons
listenit
Mar 26, 2025 · 6 min read
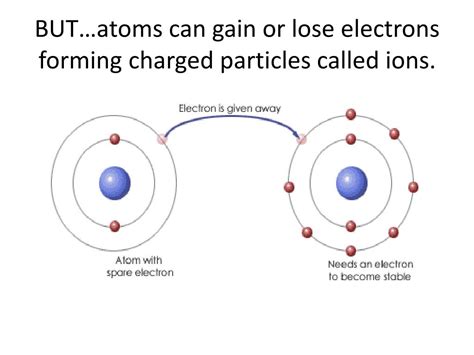
Table of Contents
Why Do Atoms Lose or Gain Electrons? Understanding Ionization and Chemical Bonding
Atoms, the fundamental building blocks of matter, are rarely content existing in isolation. Their behavior, particularly their tendency to lose or gain electrons, is central to understanding chemistry, the science of matter and its transformations. This article delves deep into the reasons behind this fundamental atomic process, exploring the concepts of ionization energy, electron affinity, and the crucial role it plays in forming chemical bonds and shaping the properties of matter.
The Structure of an Atom: A Quick Recap
Before diving into the intricacies of electron loss and gain, let's briefly review the basic structure of an atom. An atom consists of a dense, central nucleus containing positively charged protons and neutral neutrons. Surrounding this nucleus is a cloud of negatively charged electrons, arranged in specific energy levels or shells. These shells are not randomly populated; they follow a defined order, with each shell having a maximum capacity of electrons. The outermost shell, known as the valence shell, plays a crucial role in determining an atom's chemical behavior. The number of electrons in the valence shell dictates how readily an atom will lose, gain, or share electrons to achieve a stable configuration.
Ionization Energy: The Energy Cost of Losing an Electron
The tendency of an atom to lose an electron is directly related to its ionization energy. Ionization energy is defined as the minimum energy required to remove an electron from a neutral gaseous atom in its ground state. This energy must overcome the electrostatic attraction between the positively charged nucleus and the negatively charged electron. The closer an electron is to the nucleus and the greater the positive charge of the nucleus, the higher the ionization energy. Therefore, atoms with smaller atomic radii and higher nuclear charges generally have higher ionization energies.
Factors Affecting Ionization Energy:
- Nuclear Charge: A higher nuclear charge (more protons) increases the attraction for electrons, resulting in a higher ionization energy.
- Atomic Radius: A smaller atomic radius means the electrons are closer to the nucleus, experiencing a stronger electrostatic attraction and higher ionization energy.
- Shielding Effect: Inner electrons shield outer electrons from the full positive charge of the nucleus, reducing the effective nuclear charge felt by the outer electrons and lowering ionization energy.
- Electron Configuration: A stable electron configuration (e.g., a full valence shell) requires more energy to remove an electron than an unstable configuration.
Trends in Ionization Energy across the Periodic Table:
Ionization energy generally increases across a period (from left to right) due to increasing nuclear charge and decreasing atomic radius. It generally decreases down a group (from top to bottom) due to increasing atomic radius and increased shielding effect.
Electron Affinity: The Energy Released from Gaining an Electron
In contrast to ionization energy, electron affinity represents the energy change that occurs when an atom gains an electron. It is the energy released (exothermic process) or absorbed (endothermic process) when a neutral gaseous atom in its ground state acquires an electron. A high electron affinity indicates a strong tendency for an atom to gain an electron. This is often associated with atoms that are one electron short of a stable, filled electron shell.
Factors Affecting Electron Affinity:
- Nuclear Charge: A higher nuclear charge attracts the incoming electron more strongly, leading to a more negative (exothermic) electron affinity.
- Atomic Radius: A smaller atomic radius means the incoming electron is closer to the nucleus, experiencing a stronger electrostatic attraction and a more negative electron affinity.
- Electron-Electron Repulsion: The presence of existing electrons in the valence shell can repel the incoming electron, making it less likely to be accepted and reducing the electron affinity.
Trends in Electron Affinity across the Periodic Table:
Electron affinity trends are less predictable than ionization energy trends. However, in general, electron affinity tends to increase across a period (from left to right) and shows no consistent trend down a group.
The Octet Rule and Stable Electron Configurations
The driving force behind atoms losing or gaining electrons is the pursuit of a stable electron configuration. The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a full valence shell of eight electrons, similar to the stable electron configuration of noble gases. This stable configuration minimizes the atom's energy, making it less reactive.
Atoms with few valence electrons (typically metals) tend to lose electrons to achieve a stable configuration, forming positively charged ions called cations. Atoms with many valence electrons (typically nonmetals) tend to gain electrons to achieve a stable configuration, forming negatively charged ions called anions.
Chemical Bonding: The Result of Electron Transfer or Sharing
The loss and gain of electrons is not an isolated phenomenon; it is fundamentally linked to the formation of chemical bonds. Chemical bonds are forces that hold atoms together in molecules and compounds. There are two main types of chemical bonds that result from electron interactions:
1. Ionic Bonds: Electron Transfer
Ionic bonds form when there is a significant difference in electronegativity between two atoms. Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond. A highly electronegative atom (such as a nonmetal) will strongly attract electrons from a less electronegative atom (such as a metal). This leads to the transfer of electrons from the metal atom to the nonmetal atom, forming oppositely charged ions (cations and anions). These ions are then held together by strong electrostatic forces, forming an ionic bond. Example: NaCl (sodium chloride), where sodium loses an electron to become Na⁺ and chlorine gains an electron to become Cl⁻.
2. Covalent Bonds: Electron Sharing
Covalent bonds form when atoms share electrons to achieve a stable electron configuration. This often occurs between nonmetals with similar electronegativities. Instead of transferring electrons, atoms share one or more pairs of electrons to complete their valence shells. The shared electrons are attracted to the nuclei of both atoms, holding them together. Example: H₂ (hydrogen gas), where two hydrogen atoms share a pair of electrons to achieve a stable duet (two electrons in the valence shell).
Exceptions to the Octet Rule
While the octet rule serves as a useful guideline, it's not without exceptions. Some atoms can have stable configurations with less than eight electrons in their valence shell (e.g., boron with six electrons) or more than eight electrons (e.g., phosphorus and sulfur in some compounds). These exceptions often involve atoms with empty or partially filled d orbitals that can participate in bonding.
Conclusion: The Significance of Electron Transfer in Nature
The loss and gain of electrons by atoms are fundamental processes underpinning the vast majority of chemical reactions and interactions observed in the natural world. From the formation of simple salts to the intricate complexities of biological molecules, the driving force behind these processes is the quest for electronic stability. Understanding ionization energy, electron affinity, and their relationship to chemical bonding is essential for comprehending the properties of matter, the behaviour of chemical reactions, and the overall workings of the physical universe. The seemingly simple act of an atom losing or gaining an electron has profound consequences, shaping the world around us in countless ways.
Latest Posts
Latest Posts
-
What Is The Fraction Of 0 875
Mar 29, 2025
-
Copper And Silver Nitrate Balanced Equation
Mar 29, 2025
-
What Is 3 Out Of 12 As A Percentage
Mar 29, 2025
-
What Are The Common Factors Of 8 And 24
Mar 29, 2025
-
What Are The Three Main Types Of Elements
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Why Do Atoms Lose Or Gain Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
