What Are The Three Main Types Of Elements
listenit
Mar 29, 2025 · 6 min read
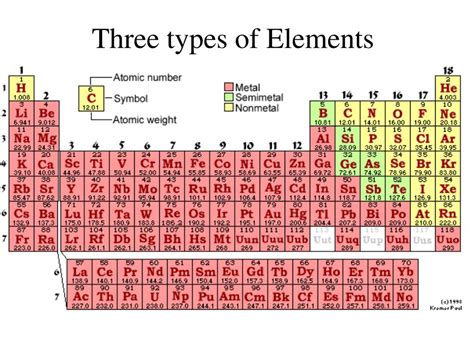
Table of Contents
- What Are The Three Main Types Of Elements
- Table of Contents
- What Are the Three Main Types of Elements? A Deep Dive into Metals, Nonmetals, and Metalloids
- 1. Metals: The Kings of Conductivity
- Defining Characteristics of Metals:
- Key Examples of Metals and their Applications:
- 2. Nonmetals: A Diverse Group with Varied Properties
- Defining Characteristics of Nonmetals:
- Key Examples of Nonmetals and their Applications:
- 3. Metalloids: Bridging the Gap
- Defining Characteristics of Metalloids:
- Key Examples of Metalloids and their Applications:
- The Importance of Understanding Element Classification
- Latest Posts
- Latest Posts
- Related Post
What Are the Three Main Types of Elements? A Deep Dive into Metals, Nonmetals, and Metalloids
The periodic table, that iconic chart adorning countless science classrooms, organizes over 100 known elements. But understanding the sheer number of elements isn't enough. To truly grasp their behavior and properties, we need to classify them. While numerous classification schemes exist, the most fundamental and widely used divides elements into three main categories: metals, nonmetals, and metalloids. This article will delve deep into each category, exploring their distinctive characteristics, key examples, and practical applications.
1. Metals: The Kings of Conductivity
Metals, the most numerous category of elements, dominate the left and center of the periodic table. Their defining characteristics stem from their unique atomic structure and the way their electrons behave. Think of the shiny, ductile, and malleable materials commonly associated with the term "metal"—that's because these properties are directly linked to their electron configuration.
Defining Characteristics of Metals:
-
Conductivity: This is arguably the most defining characteristic. Metals are excellent conductors of both electricity and heat. This is due to the presence of loosely held valence electrons, which can move freely throughout the metallic structure, allowing for easy transfer of charge and energy. This is why copper is used extensively in electrical wiring and aluminum in cookware.
-
Malleability and Ductility: Metals can be easily hammered into sheets (malleability) and drawn into wires (ductility). This ability to deform without breaking is a result of the metallic bonding, where atoms are held together by a "sea" of delocalized electrons, allowing layers of atoms to slide past each other.
-
Luster: Metals generally possess a characteristic metallic luster or shine. This is due to the interaction of light with the free electrons in the metal lattice. The light waves are absorbed and re-emitted, giving them their shiny appearance.
-
High Density and Melting Points: Most metals have relatively high densities and melting points compared to nonmetals. This is again related to the strong metallic bonding. The stronger the bond, the more energy is required to break it, resulting in a higher melting point.
-
Hardness: While some metals are softer (like sodium), many are relatively hard, contributing to their use in structural applications. The hardness varies considerably depending on the specific metal and its alloying elements.
Key Examples of Metals and their Applications:
-
Iron (Fe): A cornerstone of modern civilization, iron is used in countless applications, from construction (steel) to automobiles and machinery. Its strength and abundance make it incredibly versatile.
-
Aluminum (Al): Known for its lightweight yet strong properties, aluminum finds extensive use in aerospace, transportation, and packaging. Its resistance to corrosion also makes it a popular choice.
-
Copper (Cu): An exceptional conductor of electricity and heat, copper is indispensable in electrical wiring, plumbing, and various industrial processes.
-
Gold (Au) and Silver (Ag): These precious metals are prized for their inertness, beauty, and excellent conductivity, making them suitable for jewelry, electronics, and investments.
-
Titanium (Ti): A strong, lightweight, and corrosion-resistant metal, titanium finds applications in aerospace, medical implants, and sporting goods.
2. Nonmetals: A Diverse Group with Varied Properties
Unlike the uniform characteristics of metals, nonmetals exhibit a far greater diversity in their properties. They are typically located on the right side of the periodic table and generally lack the metallic properties described above.
Defining Characteristics of Nonmetals:
-
Poor Conductors: Nonmetals are generally poor conductors of both heat and electricity. This is because their electrons are tightly bound to their atoms, limiting their mobility. Exceptions exist, such as graphite (a form of carbon).
-
Brittle: Solid nonmetals are often brittle and tend to shatter when struck. This is because their bonding structures are less flexible than those of metals.
-
Low Density and Melting Points: Nonmetals generally have lower densities and melting points compared to metals.
-
Dull Appearance: Nonmetals typically lack the metallic luster; they often appear dull or have a variety of colors.
-
Varying States: Nonmetals can exist in all three states of matter at room temperature: solid (carbon, sulfur), liquid (bromine), and gas (oxygen, nitrogen).
Key Examples of Nonmetals and their Applications:
-
Oxygen (O): Essential for respiration and combustion, oxygen is crucial for life and industrial processes.
-
Nitrogen (N): A major component of the atmosphere, nitrogen is used in fertilizers and various industrial applications.
-
Carbon (C): A versatile element found in various allotropes (different structural forms) such as diamond, graphite, and fullerenes. It's fundamental to organic chemistry and is used in various materials.
-
Chlorine (Cl): A highly reactive halogen used in water purification and various chemical processes.
-
Sulfur (S): Used in the production of sulfuric acid, a vital industrial chemical, sulfur also has applications in fertilizers and vulcanization of rubber.
-
Hydrogen (H): The lightest element, hydrogen is used as a fuel source and in various chemical processes. It is increasingly being investigated as a clean energy source.
3. Metalloids: Bridging the Gap
Metalloids, also known as semimetals, occupy a fascinating middle ground between metals and nonmetals. They are located along the "staircase" line that separates metals from nonmetals on the periodic table. This strategic positioning gives them a unique set of properties that blend characteristics of both metals and nonmetals.
Defining Characteristics of Metalloids:
-
Semiconductors: This is the most significant characteristic of metalloids. They are semiconductors, meaning their electrical conductivity lies between that of metals (good conductors) and nonmetals (poor conductors). Their conductivity can be controlled by adding impurities (doping), making them crucial in electronics.
-
Variable Properties: Metalloids exhibit properties that are intermediate between metals and nonmetals. Their behavior can depend on factors like temperature and the presence of impurities.
-
Brittleness: Like nonmetals, metalloids are generally brittle.
Key Examples of Metalloids and their Applications:
-
Silicon (Si): The most important metalloid, silicon is the foundation of the semiconductor industry. It's used in computer chips, solar cells, and various other electronic components.
-
Germanium (Ge): Similar to silicon, germanium was once crucial in semiconductors but has been largely replaced by silicon. It still finds applications in specialized electronics and optical fibers.
-
Arsenic (As): Used in small quantities in alloys and as a doping agent in semiconductors, arsenic also has applications in pesticides (though its use is increasingly restricted due to toxicity concerns).
-
Boron (B): Used in various alloys and glasses, boron also finds applications in nuclear reactors and as a doping agent in semiconductors.
-
Antimony (Sb): Used in alloys to increase hardness and improve their properties, antimony also finds applications in flame retardants and certain types of batteries.
The Importance of Understanding Element Classification
Understanding the three main types of elements—metals, nonmetals, and metalloids—is fundamental to comprehending chemical behavior and the properties of matter. This classification scheme provides a framework for predicting how elements will react with each other, what kinds of compounds they will form, and what their potential applications might be. From the construction of skyscrapers to the fabrication of microchips, our modern world relies heavily on the unique properties of these different elemental classes. The continued research and development in materials science further underscores the importance of understanding these classifications and their nuances. The ongoing exploration of new materials and technologies hinges on our capacity to manipulate and utilize the unique characteristics of each elemental type to create innovative solutions for the challenges we face. This categorization isn't simply an academic exercise; it's the foundation of countless innovations and advancements shaping our lives every day. Further study into the intricacies of each element type will continue to unlock new possibilities and improve existing technologies.
Latest Posts
Latest Posts
-
1 3 Divided By 1 6 As A Fraction
Apr 02, 2025
-
How To Determine Zeros Of A Function
Apr 02, 2025
-
What Are Four Principles Of Natural Selection
Apr 02, 2025
-
What Is 9 To The Power Of 0
Apr 02, 2025
-
Least Common Multiple Of 36 And 12
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Are The Three Main Types Of Elements . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
