What Does Low Specific Heat Mean
listenit
Mar 26, 2025 · 5 min read
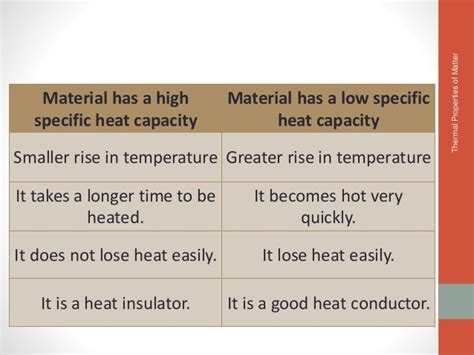
Table of Contents
What Does Low Specific Heat Mean? Understanding and Applying This Crucial Property
Specific heat capacity, often shortened to specific heat, is a fundamental physical property that dictates how much heat energy is required to raise the temperature of a substance. Understanding what low specific heat means is crucial across various scientific fields and everyday applications. This in-depth exploration will delve into the definition, implications, and practical examples of materials with low specific heat.
Defining Specific Heat: The Energy Absorbed Per Degree
Before exploring low specific heat, let's establish a firm understanding of the concept itself. Specific heat is the amount of heat required to raise the temperature of one gram (or one kilogram, depending on the units used) of a substance by one degree Celsius (or one Kelvin). It's expressed in units of Joules per gram-degree Celsius (J/g°C) or Joules per kilogram-degree Kelvin (J/kg·K). Crucially, it's an intensive property, meaning it doesn't depend on the amount of substance present. A small sample and a large sample of copper will both have the same specific heat.
The equation governing specific heat is:
Q = mcΔT
Where:
- Q represents the heat energy transferred (in Joules)
- m represents the mass of the substance (in grams or kilograms)
- c represents the specific heat capacity of the substance (in J/g°C or J/kg·K)
- ΔT represents the change in temperature (in °C or K)
This equation highlights the direct relationship between heat energy absorbed and the temperature change. A substance with a high specific heat requires a significant amount of energy to increase its temperature, while a substance with a low specific heat requires relatively little.
Understanding Low Specific Heat: Implications and Characteristics
A material with a low specific heat means it requires only a small amount of heat energy to cause a noticeable temperature increase. This implies several key characteristics:
-
Rapid Temperature Changes: Materials with low specific heat heat up and cool down quickly. This is because they readily absorb and release heat energy with minimal temperature change.
-
Efficient Heat Transfer: Their ability to absorb and release heat quickly makes them efficient in applications involving heat transfer.
-
Susceptibility to Temperature Fluctuations: Because they react rapidly to heat changes, they are more vulnerable to environmental temperature variations.
-
Lower Thermal Inertia: They have lower thermal inertia, meaning they respond rapidly to changes in heat input.
-
Potential for Thermal Shock: While rapid heating and cooling can be beneficial in certain situations, rapid temperature changes can also lead to thermal shock, especially in materials with low thermal conductivity.
Materials with Low Specific Heat: Examples and Applications
Various materials exhibit low specific heat. Let's explore some examples and their applications:
1. Metals (Many):
Many metals, particularly those with simple crystal structures, possess relatively low specific heats. For example, iron, copper, and aluminum all have relatively low specific heat values compared to water.
Applications: Their rapid heating and cooling properties make them suitable for applications like cookware (where fast heating is desired), heat sinks in electronics (to dissipate heat effectively), and industrial processes requiring quick temperature adjustments.
2. Mercury:
Mercury is a liquid metal renowned for its extremely low specific heat.
Applications: Historically, mercury was used in thermometers because of its rapid temperature response, although its toxicity has led to its replacement in many applications.
3. Lead:
Lead also has a notably low specific heat.
Applications: This property contributed to its use in radiation shielding (in conjunction with its high density) and in some applications requiring weight without excessive heat retention.
4. Glass:
While specific heat varies slightly depending on composition, glass generally has relatively low specific heat compared to water.
Applications: Its low specific heat is relevant in applications where rapid heating or cooling is needed, although the fragility of glass limits its use in certain high-temperature scenarios.
5. Certain Plastics:
Many plastics, like polypropylene and polystyrene, have comparatively low specific heats.
Applications: Their low specific heat is an advantage in applications where rapid heating or cooling is desirable, such as in disposable food containers or certain types of packaging.
Contrast with High Specific Heat: Water as the Benchmark
Water is often cited as having a remarkably high specific heat. This means that it takes a significant amount of energy to raise the temperature of water, even a small amount. This property is crucial for several reasons:
-
Climate Regulation: Water bodies like oceans and lakes moderate climate, absorbing and releasing large amounts of heat with minimal temperature change.
-
Biological Significance: Water's high specific heat is essential for maintaining stable body temperatures in living organisms.
Practical Implications and Considerations
Understanding the concept of low specific heat is critical in diverse fields:
1. Engineering:
In engineering design, specific heat is a key factor in selecting materials for applications involving heat transfer, temperature control, and thermal management. Components in engines, electronics, and other systems often require materials with optimized specific heat properties.
2. Material Science:
Researchers in material science continually investigate and develop new materials with tailored specific heat capacities for specific applications.
3. Cooking:
In cooking, the specific heat of materials influences how quickly they heat up and how evenly they cook. The low specific heat of metals contributes to their effectiveness in cookware.
4. Climate Modeling:
In climate modeling, the specific heat of water plays a significant role in simulating global climate patterns and predicting climate change impacts.
5. Thermodynamics:
Low specific heat impacts calculations in thermodynamic processes, impacting efficiency and energy requirements.
Conclusion: Choosing the Right Material for the Job
The specific heat of a material is a crucial factor in a wide range of applications. While high specific heat can be advantageous for temperature stability and regulation, low specific heat is essential where rapid heating and cooling are desired. By carefully considering specific heat values, engineers and scientists can select materials that optimize performance and efficiency in diverse systems and processes. Understanding what low specific heat means empowers informed decision-making in various fields, leading to improved designs, more effective processes, and a deeper understanding of the physical world around us. Remember that the choice of material isn't solely dictated by specific heat; other factors like thermal conductivity, density, and cost also heavily influence the selection process. However, grasping the implications of low specific heat is a cornerstone of understanding material behavior and application.
Latest Posts
Latest Posts
-
What Are Coefficients In Chemical Equations
Mar 29, 2025
-
How Many Electrons Do Noble Gases Have
Mar 29, 2025
-
2 5 As A Percentage And Decimal
Mar 29, 2025
-
What Is The Least Common Multiple Of 6 And 14
Mar 29, 2025
-
Is The Sun Older Than Earth
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Does Low Specific Heat Mean . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
