What Is The Oxidation Number Of Sulfur In H2so4
listenit
Mar 28, 2025 · 6 min read
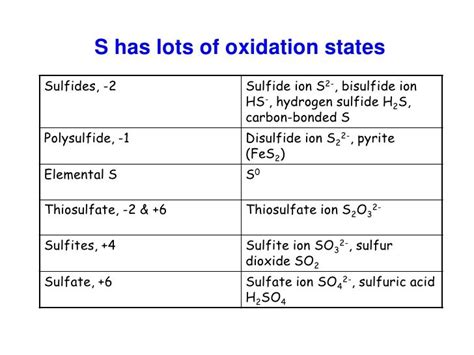
Table of Contents
What is the Oxidation Number of Sulfur in H₂SO₄? A Comprehensive Guide
Determining the oxidation number of an element within a compound is a fundamental concept in chemistry. Understanding oxidation states is crucial for balancing redox reactions, predicting chemical behavior, and comprehending the reactivity of different substances. This article delves into the process of calculating the oxidation number of sulfur in sulfuric acid (H₂SO₄), providing a comprehensive explanation suitable for both students and those looking to refresh their chemistry knowledge.
Understanding Oxidation Numbers
Before we tackle the specific case of sulfuric acid, let's establish a solid foundation in oxidation number concepts. The oxidation number, also known as the oxidation state, is a hypothetical charge assigned to an atom in a molecule or ion. It represents the number of electrons an atom has gained or lost compared to its neutral state. It's important to remember that oxidation numbers are not necessarily the real charges on atoms; they are a useful bookkeeping tool for tracking electron transfer in chemical reactions.
Several rules govern the assignment of oxidation numbers:
- Rule 1: The oxidation number of an element in its free or uncombined state is always zero. For example, the oxidation number of O₂ is 0, and the oxidation number of S₈ is 0.
- Rule 2: The oxidation number of a monatomic ion is equal to its charge. For example, the oxidation number of Na⁺ is +1, and the oxidation number of Cl⁻ is -1.
- Rule 3: The oxidation number of hydrogen is usually +1, except in metal hydrides where it is -1. Examples include H₂O (hydrogen is +1) and NaH (hydrogen is -1).
- Rule 4: The oxidation number of oxygen is usually -2, except in peroxides (where it is -1) and in compounds with fluorine (where it can be positive). Examples include H₂O (oxygen is -2) and H₂O₂ (oxygen is -1).
- Rule 5: The sum of the oxidation numbers of all atoms in a neutral molecule is zero.
- Rule 6: The sum of the oxidation numbers of all atoms in a polyatomic ion is equal to the charge of the ion.
Calculating the Oxidation Number of Sulfur in H₂SO₄
Now, let's apply these rules to determine the oxidation number of sulfur (S) in sulfuric acid (H₂SO₄).
-
Identify the known oxidation numbers: We know the oxidation number of hydrogen (H) is typically +1 and the oxidation number of oxygen (O) is typically -2.
-
Set up an algebraic equation: Let 'x' represent the oxidation number of sulfur. Using Rule 5 (the sum of oxidation numbers in a neutral molecule is zero), we can write the following equation:
2(+1) + x + 4(-2) = 0
-
Solve for x:
2 + x - 8 = 0 x - 6 = 0 x = +6
Therefore, the oxidation number of sulfur in H₂SO₄ is +6.
Significance of the +6 Oxidation State of Sulfur
The +6 oxidation state of sulfur in H₂SO₄ is significant for several reasons:
-
High Oxidation State: This is one of the highest oxidation states sulfur can achieve. This high oxidation state indicates that sulfur has lost a significant number of electrons, making it a strong oxidizing agent.
-
Acidic Nature: The high oxidation state of sulfur contributes to the strong acidic nature of sulfuric acid. The highly polarized S-O bonds make the molecule readily donate protons (H⁺ ions).
-
Reactivity: Sulfuric acid's reactivity is directly linked to the +6 oxidation state of sulfur. This high oxidation state allows it to participate in a wide range of redox reactions, acting as both an oxidizing and dehydrating agent.
-
Industrial Importance: The +6 oxidation state of sulfur in sulfuric acid is critical to its extensive industrial applications. Sulfuric acid is a cornerstone chemical used in various industries, including fertilizer production, metal refining, and petroleum processing. Its powerful oxidizing and dehydrating properties make it invaluable in these processes.
Further Exploration of Sulfur's Oxidation States
Sulfur is a versatile element capable of exhibiting a wide range of oxidation states, from -2 to +6. Understanding these different oxidation states is key to comprehending sulfur's diverse chemical behavior and its role in various compounds.
Here are some examples illustrating different oxidation states of sulfur:
- Hydrogen sulfide (H₂S): Sulfur has an oxidation number of -2.
- Sulfur dioxide (SO₂): Sulfur has an oxidation number of +4.
- Sulfur trioxide (SO₃): Sulfur has an oxidation number of +6.
- Sulfides (e.g., FeS): Sulfur typically has an oxidation number of -2.
- Sulfates (e.g., Na₂SO₄): Sulfur typically has an oxidation number of +6.
- Thiosulfates (e.g., Na₂S₂O₃): Sulfur exhibits multiple oxidation states within the same molecule.
Redox Reactions and Sulfur's Oxidation State Changes
Oxidation-reduction (redox) reactions involve the transfer of electrons between species. Sulfur, due to its variable oxidation states, readily participates in such reactions. The change in oxidation number indicates whether a sulfur-containing species has been oxidized (loss of electrons, increase in oxidation number) or reduced (gain of electrons, decrease in oxidation number).
For example, the conversion of sulfur dioxide (SO₂, sulfur oxidation state +4) to sulfuric acid (H₂SO₄, sulfur oxidation state +6) is an oxidation reaction. Conversely, the reduction of sulfuric acid to sulfur dioxide is a reduction reaction.
Practical Applications and Industrial Relevance
The ability to determine oxidation numbers has numerous practical applications, particularly in industrial settings. Accurate determination of oxidation states allows chemists and engineers to:
- Balance Redox Equations: Predicting the stoichiometry of redox reactions is essential for efficient process control in industrial chemical processes.
- Design Electrochemical Cells: Understanding oxidation states is critical for designing electrochemical cells, such as batteries, which rely on electron transfer reactions.
- Predict Chemical Reactivity: Knowledge of oxidation states helps in predicting the reactivity of different compounds, aiding in material selection and process optimization.
- Analyze Environmental Samples: Determining the oxidation states of sulfur species in environmental samples is vital for monitoring air and water quality.
Conclusion
Determining the oxidation number of sulfur in H₂SO₄, which is +6, is a straightforward application of fundamental rules governing oxidation numbers. This seemingly simple calculation underpins a wealth of important chemical concepts and has far-reaching implications for understanding the reactivity, properties, and industrial applications of sulfuric acid and sulfur-containing compounds in general. A thorough understanding of oxidation numbers is essential for any chemist, whether in academia or industry. From balancing redox reactions to predicting chemical behavior, the concept remains crucial in various fields. The versatility of sulfur and its ability to exhibit a wide range of oxidation states contribute to its importance in numerous chemical processes and industrial applications.
Latest Posts
Latest Posts
-
What Is The Square Root Of 70
Mar 31, 2025
-
What Is The Current Model Of The Atom
Mar 31, 2025
-
How Many Chromosomes Does A Cow Have
Mar 31, 2025
-
What Percentage Of 50 Is 32
Mar 31, 2025
-
How Many Valence Electrons In Si
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is The Oxidation Number Of Sulfur In H2so4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
