What Is The Current Model Of The Atom
listenit
Mar 31, 2025 · 6 min read
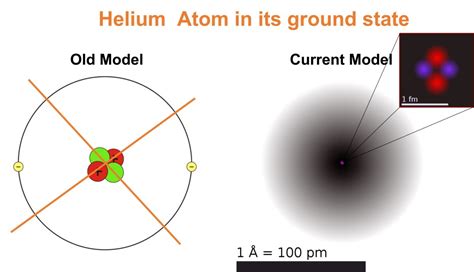
Table of Contents
What is the Current Model of the Atom?
The atom, the fundamental building block of matter, has captivated scientists for centuries. Our understanding of its structure has evolved dramatically, from ancient Greek philosophers' philosophical musings to the sophisticated quantum mechanical models of today. This article dives deep into the current understanding of the atomic model, exploring its components, behaviors, and the ongoing research shaping our knowledge.
From Democritus to Quantum Mechanics: A Brief History
The concept of the atom dates back to ancient Greece, where philosophers like Democritus proposed the idea of indivisible particles called "atomos." However, this was purely a philosophical concept, lacking experimental evidence. It wasn't until the late 19th and early 20th centuries that scientific advancements provided the tools to probe the atom's internal structure.
Key milestones in the development of atomic models include:
-
Dalton's Atomic Model (early 1800s): John Dalton proposed that atoms were solid, indivisible spheres, differing in mass and properties for different elements. This marked the beginning of a scientific approach to understanding atoms.
-
Thomson's Plum Pudding Model (1904): J.J. Thomson's discovery of the electron, a negatively charged subatomic particle, led to the "plum pudding" model. This model depicted the atom as a positively charged sphere with negatively charged electrons embedded within it, like plums in a pudding.
-
Rutherford's Nuclear Model (1911): Ernest Rutherford's gold foil experiment revolutionized atomic theory. The experiment revealed a dense, positively charged nucleus at the atom's center, with electrons orbiting it at a distance. This model significantly changed the perception of the atom from a uniform sphere to a largely empty space with a central nucleus.
-
Bohr's Model (1913): Niels Bohr refined Rutherford's model by incorporating quantum theory. He proposed that electrons orbit the nucleus in specific energy levels or shells, and electrons can jump between these levels by absorbing or emitting energy. This model successfully explained the spectral lines of hydrogen but failed to accurately predict the behavior of more complex atoms.
-
The Quantum Mechanical Model (1920s-present): The current model of the atom is based on quantum mechanics, a sophisticated mathematical framework describing the behavior of matter at the atomic and subatomic levels. This model rejects the concept of precisely defined electron orbits, instead describing electrons as existing in probability clouds or orbitals.
The Quantum Mechanical Model: A Deeper Dive
The quantum mechanical model is the most accurate and comprehensive representation of the atom we have today. It's based on several key principles:
-
Wave-Particle Duality: Electrons exhibit both wave-like and particle-like properties. This means they can behave like waves, exhibiting interference and diffraction, but also like particles, possessing mass and charge.
-
Heisenberg's Uncertainty Principle: This principle states that it's impossible to simultaneously know both the precise position and momentum of an electron. The more accurately we know one, the less accurately we know the other. This inherent uncertainty limits our ability to predict an electron's exact path.
-
Quantum Numbers: Each electron in an atom is described by a set of four quantum numbers:
- Principal Quantum Number (n): This number determines the electron's energy level and average distance from the nucleus. Higher values of 'n' correspond to higher energy levels and greater distances.
- Azimuthal Quantum Number (l): This number specifies the electron's subshell or orbital shape (s, p, d, f). 'l' can range from 0 to n-1.
- Magnetic Quantum Number (ml): This number describes the orientation of the orbital in space. 'ml' can range from -l to +l.
- Spin Quantum Number (ms): This number describes the intrinsic angular momentum, or spin, of the electron. It can have a value of +1/2 or -1/2.
-
Orbitals: Orbitals are regions of space around the nucleus where there's a high probability of finding an electron. They're not fixed paths like in Bohr's model, but rather probability distributions. The shape and orientation of orbitals depend on the quantum numbers. For example, 's' orbitals are spherical, 'p' orbitals are dumbbell-shaped, and 'd' and 'f' orbitals have more complex shapes.
-
Electron Configuration: The electron configuration describes how electrons are distributed among the various energy levels and orbitals within an atom. It follows the Aufbau principle (filling lower energy levels first), Hund's rule (maximizing unpaired electrons in a subshell), and the Pauli exclusion principle (no two electrons can have the same four quantum numbers).
Subatomic Particles: Protons, Neutrons, and Electrons
The atom consists of three fundamental subatomic particles:
-
Protons: Positively charged particles located in the nucleus. The number of protons determines an element's atomic number and its identity.
-
Neutrons: Neutral particles (no charge) also found in the nucleus. Neutrons contribute to an atom's mass but not its charge. The number of neutrons can vary within an element, leading to isotopes.
-
Electrons: Negatively charged particles that orbit the nucleus in orbitals. Electrons are much lighter than protons and neutrons. The number of electrons in a neutral atom equals the number of protons.
Isotopes and Ions
-
Isotopes: Atoms of the same element with the same number of protons but a different number of neutrons. Isotopes have the same atomic number but different mass numbers (protons + neutrons). Some isotopes are stable, while others are radioactive and decay over time.
-
Ions: Atoms that have gained or lost electrons, resulting in a net electrical charge. Cations are positively charged ions (lost electrons), and anions are negatively charged ions (gained electrons).
Atomic Properties and Periodic Trends
The quantum mechanical model helps explain many of the periodic trends observed in the periodic table of elements, such as:
-
Atomic Radius: The size of an atom generally increases down a group (column) and decreases across a period (row).
-
Ionization Energy: The energy required to remove an electron from an atom. Ionization energy generally increases across a period and decreases down a group.
-
Electronegativity: The ability of an atom to attract electrons in a chemical bond. Electronegativity generally increases across a period and decreases down a group.
Advanced Concepts and Ongoing Research
The quantum mechanical model, while remarkably successful, is still being refined and extended. Ongoing research areas include:
-
Quantum Electrodynamics (QED): A theory that combines quantum mechanics and special relativity to describe the interaction of light and matter at the atomic level.
-
Quantum Chromodynamics (QCD): A theory describing the strong force that holds quarks together to form protons and neutrons.
-
Exploring Exotic Atoms: Studies of atoms with unusual compositions, such as antimatter atoms or atoms with muons instead of electrons, provide insights into fundamental physics.
-
Quantum Computing: Harnessing the principles of quantum mechanics to develop powerful new computers.
Conclusion
The current model of the atom, based on quantum mechanics, is a testament to the power of scientific inquiry. While the model provides a highly accurate and detailed picture of atomic structure and behavior, research continues to refine and expand our understanding. From the fundamental particles within the atom to its interactions with light and other matter, the atom remains a fascinating subject of study, with its secrets gradually being revealed through ongoing scientific advancements. The journey from Democritus' philosophical atom to the sophisticated quantum mechanical model is a testament to human curiosity and the power of scientific investigation, a journey that undoubtedly continues. Understanding the current model of the atom is fundamental to comprehending chemistry, physics, and many other scientific disciplines. The concepts discussed here, while complex, provide a crucial foundation for deeper exploration into the wonders of the subatomic world.
Latest Posts
Latest Posts
-
What Are Three Parts Of Atp Molecule
Apr 01, 2025
-
How Many Right Angles Does A Quadrilateral Have
Apr 01, 2025
-
Is Magnesium A Gas Liquid Or Solid
Apr 01, 2025
-
Where In The Chloroplast Do The Light Reactions Take Place
Apr 01, 2025
-
What Is All The Colors Combined
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is The Current Model Of The Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
