What Is The Molecular Shape Of Pf3
listenit
Mar 26, 2025 · 6 min read
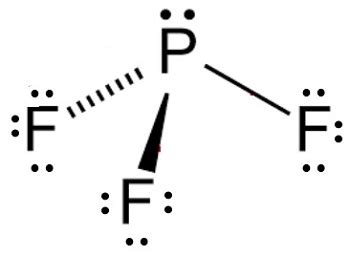
Table of Contents
What is the Molecular Shape of PF3? A Deep Dive into Phosphorus Trifluoride Geometry
Phosphorus trifluoride (PF3) is a fascinating inorganic compound with a unique molecular shape that significantly influences its properties and reactivity. Understanding its geometry is crucial for grasping its role in various chemical processes and applications. This article provides a comprehensive exploration of the molecular shape of PF3, delving into the underlying principles of valence shell electron pair repulsion (VSEPR) theory, hybridization, and bond angles. We'll also examine the consequences of this shape on PF3's physical and chemical characteristics.
Understanding VSEPR Theory: The Foundation of Molecular Geometry
Before diving into the specifics of PF3, let's establish a strong foundation in VSEPR theory. This cornerstone of molecular geometry prediction states that the electron pairs surrounding a central atom will arrange themselves to minimize repulsion. This arrangement dictates the overall shape of the molecule. The key factors considered are:
- Lone pairs: Non-bonding electron pairs on the central atom. These exert stronger repulsive forces than bonding pairs.
- Bonding pairs: Electron pairs shared between the central atom and surrounding atoms.
- Number of electron domains: The total number of lone pairs and bonding pairs around the central atom.
The VSEPR theory predicts several common molecular geometries based on the number of electron domains: linear (2 domains), trigonal planar (3 domains), tetrahedral (4 domains), trigonal bipyramidal (5 domains), and octahedral (6 domains). However, the presence of lone pairs can distort these ideal geometries.
Determining the Molecular Shape of PF3: Applying VSEPR
Now, let's apply VSEPR theory to phosphorus trifluoride (PF3).
1. Lewis Structure of PF3
The Lewis structure of PF3 shows phosphorus (P) as the central atom, bonded to three fluorine (F) atoms. Phosphorus has 5 valence electrons, and each fluorine atom contributes 1 valence electron, resulting in a total of 8 valence electrons. The Lewis structure is:
F
|
F - P - F
|
F
2. Electron Domains and Geometry
In PF3, the phosphorus atom is surrounded by three bonding pairs (bonds to the three fluorine atoms) and one lone pair of electrons. This gives a total of four electron domains. According to VSEPR theory, four electron domains arrange themselves in a tetrahedral geometry to minimize repulsion.
3. Molecular Shape: Trigonal Pyramidal
While the electron domains are tetrahedrally arranged, the molecular shape considers only the positions of the atoms, not the lone pair. Therefore, the molecular shape of PF3 is trigonal pyramidal. This means the three fluorine atoms form the base of a pyramid, with the phosphorus atom at the apex. The lone pair of electrons occupies a position that influences the bond angles but is not considered part of the molecular shape.
Hybridization in PF3: Understanding the Bonding
The bonding in PF3 is best explained by considering the hybridization of the phosphorus atom. Phosphorus's ground state electron configuration is [Ne] 3s² 3p³. To form four bonds (three with fluorine and one with the lone pair), one 3s electron is promoted to the empty 3d orbital. This results in the hybridization of one 3s and three 3p orbitals, forming four sp³ hybrid orbitals.
These sp³ hybrid orbitals are arranged tetrahedrally around the phosphorus atom, each accommodating one electron pair. Three of these hybrid orbitals overlap with the p orbitals of the fluorine atoms to form three sigma (σ) bonds. The remaining sp³ hybrid orbital houses the lone pair of electrons.
This hybridization explains the tetrahedral arrangement of electron domains and the trigonal pyramidal molecular shape.
Bond Angles in PF3: Deviations from Ideal Geometry
In an ideal tetrahedral structure, the bond angles are 109.5°. However, in PF3, the bond angles are slightly less than this ideal value, typically around 96-98°. This deviation is attributed to the presence of the lone pair of electrons on the phosphorus atom.
Lone pairs exert stronger repulsive forces than bonding pairs. Therefore, the lone pair pushes the bonding pairs closer together, resulting in a compression of the F-P-F bond angles. The stronger electronegativity of fluorine compared to phosphorus also contributes to this effect.
Consequences of the Trigonal Pyramidal Shape: Physical and Chemical Properties
The trigonal pyramidal shape of PF3 has profound consequences for its physical and chemical properties:
-
Polarity: Due to its asymmetrical shape and the difference in electronegativity between phosphorus and fluorine, PF3 is a polar molecule. This polarity leads to stronger intermolecular forces compared to nonpolar molecules of similar size, influencing its boiling point and solubility.
-
Reactivity: The lone pair of electrons on phosphorus makes PF3 a Lewis base. This means it can donate its lone pair to form coordinate covalent bonds with Lewis acids. This Lewis basicity is exploited in various chemical reactions and catalytic processes.
-
Spectroscopic Properties: The molecular geometry influences the vibrational and rotational spectra of PF3, providing valuable information about its structure and bonding through techniques like infrared (IR) and Raman spectroscopy.
-
Applications: PF3 has found applications in various fields, including:
- Organometallic chemistry: As a ligand in transition metal complexes.
- Semiconductor industry: In the production of certain electronic materials.
- Chemical synthesis: As a reactant or catalyst in specific chemical reactions.
Comparing PF3 to other Phosphorous Halides
It's helpful to compare PF3 with other phosphorus halides to understand how the molecular shape influences the properties. For instance, PCl3 also exhibits a trigonal pyramidal shape due to the lone pair on phosphorus. However, the larger size of chlorine atoms leads to slightly different bond angles and slightly weaker polarity compared to PF3. On the other hand, PF5 adopts a trigonal bipyramidal geometry with five bonding pairs and no lone pairs. This demonstrates how the number of electron domains significantly affects the molecular shape.
Advanced Topics: Exploring Further Aspects of PF3's Structure
The discussion above provides a foundational understanding of the molecular shape of PF3. For a more in-depth analysis, we can delve into:
-
Computational Chemistry: Advanced computational methods, such as density functional theory (DFT), can provide highly accurate predictions of bond lengths, bond angles, and other structural parameters, offering a more detailed view of PF3's electronic structure.
-
Vibrational Spectroscopy: Detailed analysis of the vibrational modes of PF3 through IR and Raman spectroscopy can confirm the predicted geometry and provide insights into the force constants of the P-F bonds.
-
X-ray Crystallography: Experimental techniques like X-ray crystallography provide experimental confirmation of the predicted molecular shape and bond angles in the solid state.
-
Influence of Temperature and Pressure: The molecular geometry and bond angles might exhibit subtle changes under different temperature and pressure conditions, demanding exploration using advanced experimental and computational techniques.
Conclusion: The Importance of Understanding PF3's Molecular Shape
Understanding the molecular shape of PF3 is paramount for predicting and interpreting its physical and chemical properties. The trigonal pyramidal geometry, a direct consequence of VSEPR theory and sp³ hybridization, governs its polarity, reactivity as a Lewis base, and its applications in various fields. This example showcases the crucial role of molecular geometry in understanding the behavior of molecules and their interactions in chemical systems. Further exploration of PF3's structure using advanced techniques continues to refine our knowledge of this intriguing compound and contributes to the wider field of molecular science.
Latest Posts
Latest Posts
-
What Does Atomic Number Of An Element Represent
Mar 26, 2025
-
What Is The Electron Configuration For Chlorine
Mar 26, 2025
-
A Cyclist Accelerates From 0m S To 8
Mar 26, 2025
-
What Is The Conjugate Acid Of H2so4
Mar 26, 2025
-
How Much Is One Degree Celsius
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molecular Shape Of Pf3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
