What Does Atomic Number Of An Element Represent
listenit
Mar 26, 2025 · 5 min read
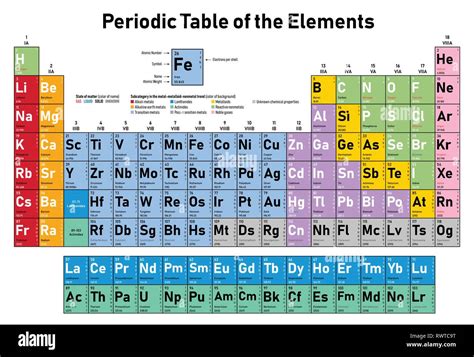
Table of Contents
What Does the Atomic Number of an Element Represent?
The atomic number of an element is a fundamental concept in chemistry and physics, representing a cornerstone of our understanding of matter. It's more than just a number; it's a key that unlocks a wealth of information about an element's properties, behavior, and place within the periodic table. This article delves deep into the meaning of the atomic number, exploring its significance in various scientific contexts and highlighting its crucial role in shaping our comprehension of the universe.
Understanding the Atom: The Foundation of Atomic Number
Before we delve into the intricacies of atomic numbers, it's crucial to establish a basic understanding of the atom itself. An atom is the smallest unit of an element that retains the chemical properties of that element. It's composed of three fundamental subatomic particles:
- Protons: Positively charged particles located within the atom's nucleus.
- Neutrons: Neutral particles (no charge) also residing in the nucleus.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels.
The Atomic Number: The Defining Characteristic
The atomic number (Z) of an element is simply the number of protons found in the nucleus of an atom of that element. This number is unique to each element and is what fundamentally distinguishes one element from another. No two elements share the same atomic number. For example:
- Hydrogen (H): Atomic number 1 (1 proton)
- Helium (He): Atomic number 2 (2 protons)
- Oxygen (O): Atomic number 8 (8 protons)
- Gold (Au): Atomic number 79 (79 protons)
This seemingly simple definition has profound implications. The atomic number dictates:
1. The Element's Identity: The Unique Fingerprint
The atomic number acts as an element's unique identifier. Just as a fingerprint uniquely identifies an individual, the atomic number unequivocally identifies a chemical element. This is because the number of protons determines the element's fundamental chemical properties. Changing the number of protons fundamentally changes the element itself.
2. The Number of Electrons in a Neutral Atom: Balancing the Charges
In a neutral atom (an atom with no overall electrical charge), the number of electrons equals the number of protons. This balance of positive and negative charges ensures the atom is electrically stable. However, atoms can gain or lose electrons to form ions, resulting in a net positive or negative charge. While the number of electrons can change, the number of protons (and therefore the atomic number) remains constant.
3. The Element's Position in the Periodic Table: Organizing the Elements
The periodic table organizes elements based on their atomic number and recurring chemical properties. Elements are arranged in order of increasing atomic number, reflecting the systematic increase in the number of protons. This organization reveals patterns in electron configurations and, consequently, the elements' chemical behavior. Elements within the same group (vertical column) exhibit similar chemical properties due to similar outer electron configurations, which are directly influenced by the atomic number.
4. The Element's Chemical Properties: Dictating Reactivity
The atomic number indirectly determines an element's chemical properties. The number of protons dictates the number of electrons, and the arrangement of these electrons in electron shells determines how the atom will interact with other atoms. Electrons in the outermost shell, known as valence electrons, are particularly crucial in determining an element's reactivity. Elements with similar numbers of valence electrons tend to exhibit similar chemical behavior.
5. Isotopes and Atomic Mass: Variations within an Element
While the atomic number defines the element, the number of neutrons can vary. Atoms of the same element with differing numbers of neutrons are called isotopes. Isotopes have the same atomic number (same number of protons) but different atomic masses (due to the varying number of neutrons). For example, carbon has two common isotopes: carbon-12 (6 protons, 6 neutrons) and carbon-14 (6 protons, 8 neutrons). Both are carbon because they both have 6 protons (atomic number 6), but their atomic masses differ due to the differing number of neutrons.
The Significance of Atomic Number in Different Fields
The concept of atomic number extends beyond basic chemistry, playing a crucial role in various scientific disciplines:
1. Nuclear Physics: Understanding Nuclear Reactions
In nuclear physics, the atomic number is fundamental to understanding nuclear reactions. Nuclear reactions involve changes in the nucleus, often altering the number of protons and neutrons. Nuclear fission and fusion processes, for example, involve significant changes in atomic numbers as atoms are split or combined. These processes are central to understanding nuclear energy and the formation of elements in stars.
2. Astrophysics: Stellar Nucleosynthesis
Astrophysics utilizes atomic numbers to understand the processes of stellar nucleosynthesis. This is the creation of new atomic nuclei in the cores of stars through nuclear fusion. By studying the abundance of elements with specific atomic numbers in stars and celestial objects, scientists can gain insights into the processes that have shaped the universe.
3. Material Science: Designing New Materials
Material scientists use atomic numbers to design and synthesize new materials with specific properties. By understanding how the atomic numbers of constituent elements influence the material's structure and behavior, scientists can create materials with enhanced strength, conductivity, or other desired characteristics. This is crucial in developing advanced technologies.
4. Analytical Chemistry: Identifying Unknown Substances
Analytical chemists utilize atomic numbers in various techniques to identify unknown substances. Techniques such as atomic absorption spectroscopy and X-ray fluorescence spectroscopy rely on the unique interaction of elements with specific wavelengths of light or X-rays, providing information about the element's atomic number and concentration in a sample.
Conclusion: The Universal Significance of Atomic Number
The atomic number of an element is not merely a numerical designation; it represents the fundamental identity and properties of an element. This single number unlocks a wealth of information about an element's chemical behavior, its position in the periodic table, and its role in various scientific fields. From the smallest atoms to the largest stars, the atomic number plays a pivotal role in our understanding of the universe and the materials that comprise it. Its significance is truly universal, making it a cornerstone of modern science and technology. Understanding the concept of the atomic number provides a foundation for further exploration into the fascinating world of chemistry and physics.
Latest Posts
Latest Posts
-
Is Ml And Mg The Same
Mar 29, 2025
-
How To Write In Logarithmic Form
Mar 29, 2025
-
What Is A 24 Out Of 35
Mar 29, 2025
-
How Many Light Years Away Is Neptune
Mar 29, 2025
-
What Is 3 4 Of A Lb
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Does Atomic Number Of An Element Represent . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
