What Is The Conjugate Acid Of H2so4
listenit
Mar 26, 2025 · 6 min read
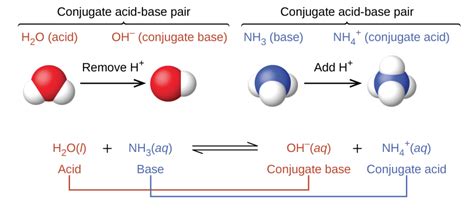
Table of Contents
What is the Conjugate Acid of H₂SO₄? Understanding Brønsted-Lowry Theory and Acid-Base Reactions
Sulfuric acid (H₂SO₄) is a strong diprotic acid, meaning it can donate two protons (H⁺) in aqueous solutions. Understanding its conjugate acid, however, requires a deeper dive into the concept of conjugate acid-base pairs within the Brønsted-Lowry theory of acids and bases. This article will explore this topic comprehensively, explaining the concept of conjugate acids, detailing the stepwise dissociation of sulfuric acid, and examining the properties of the resulting species. We'll also delve into related concepts like acid strength and the role of solvent in these reactions.
Understanding Conjugate Acid-Base Pairs
The Brønsted-Lowry theory defines an acid as a proton donor and a base as a proton acceptor. A crucial aspect of this theory is the concept of conjugate acid-base pairs. When an acid donates a proton, it forms its conjugate base. Conversely, when a base accepts a proton, it forms its conjugate acid. These pairs are related by the difference of a single proton (H⁺).
Key takeaway: A conjugate acid is formed when a base accepts a proton. Therefore, H₂SO₄, being an acid, cannot have a conjugate acid in the conventional sense. It can only have a conjugate base.
The Stepwise Dissociation of Sulfuric Acid
Sulfuric acid is a strong acid, meaning it almost completely dissociates in water. However, its diprotic nature means it dissociates in two distinct steps:
Step 1:
H₂SO₄(aq) + H₂O(l) → HSO₄⁻(aq) + H₃O⁺(aq)
In this first step, sulfuric acid donates one proton to a water molecule, forming the bisulfate ion (HSO₄⁻), which is the conjugate base of H₂SO₄, and a hydronium ion (H₃O⁺). This step is essentially complete in aqueous solution due to the high strength of sulfuric acid.
Step 2:
HSO₄⁻(aq) + H₂O(l) ⇌ H₂SO₄(aq) + OH⁻(aq)
The bisulfate ion (HSO₄⁻) is a weak acid. It can further donate a proton to another water molecule, forming sulfate ion (SO₄²⁻) and a hydronium ion (H₃O⁺). However, this second dissociation is significantly less complete than the first, represented by the equilibrium arrow (⇌). This equilibrium lies far to the left, indicating that the bisulfate ion is a weak acid and only partially dissociates.
The Conjugate Base of H₂SO₄ and its Properties
As established, the conjugate base of H₂SO₄ is the bisulfate ion (HSO₄⁻). Understanding its properties is crucial to grasping the acid-base chemistry of sulfuric acid.
-
Amphoteric Nature: The bisulfate ion is amphoteric, meaning it can act as both an acid and a base. As shown in Step 2, it can act as an acid by donating a proton. It can also act as a base by accepting a proton, for example, reacting with a strong acid like HCl:
HSO₄⁻(aq) + HCl(aq) → H₂SO₄(aq) + Cl⁻(aq)
-
Stability: The bisulfate ion is a relatively stable species in aqueous solutions. Its stability contributes to the incomplete dissociation of the second proton from sulfuric acid.
-
Acid Strength: While HSO₄⁻ is a weak acid, it is still capable of donating a proton to certain bases. Its acid dissociation constant (Ka) is considerably smaller than that of H₂SO₄, reflecting its weaker acid strength.
Why H₂SO₄ Doesn't Have a Conjugate Acid
The question of a conjugate acid for H₂SO₄ stems from a misunderstanding of the Brønsted-Lowry definition. A conjugate acid is formed when a base accepts a proton. H₂SO₄ is an acid; it donates protons. To form a conjugate acid, it would need to accept a proton. While theoretically possible under extreme conditions with a very strong acid, it is not a typical reaction for sulfuric acid. Therefore, discussing the conjugate acid of H₂SO₄ is not relevant within the typical context of acid-base chemistry.
The Role of the Solvent (Water)
The solvent, water, plays a crucial role in the dissociation of sulfuric acid. Water acts as a base, accepting protons from H₂SO₄. The high dielectric constant of water helps to stabilize the resulting ions (HSO₄⁻ and H₃O⁺), facilitating the dissociation process. The concentration of water also impacts the equilibrium position of the second dissociation step.
Implications in Different Chemical Environments
The behavior of sulfuric acid and its conjugate base can vary depending on the chemical environment. In non-aqueous solvents, the degree of dissociation can be significantly different from that observed in water. The presence of other ions or molecules can also affect the equilibrium position of the dissociation reactions.
Practical Applications and Importance
The acid-base chemistry of sulfuric acid is crucial in various applications, including:
-
Industrial Processes: Sulfuric acid is a vital industrial chemical used in the production of fertilizers, detergents, and other chemicals. Its strong acidity and ability to donate two protons make it a powerful reagent in numerous chemical processes.
-
Analytical Chemistry: The stepwise dissociation of sulfuric acid is exploited in various analytical techniques, such as titrations and pH measurements. Understanding the properties of H₂SO₄ and HSO₄⁻ is essential for accurately interpreting the results of these techniques.
-
Battery Technology: Sulfuric acid is a key component in lead-acid batteries, where its dissociation and the subsequent reactions play a crucial role in generating electrical energy.
Advanced Concepts: Leveling Effect
The leveling effect is a phenomenon where the strongest acid that can exist in a given solvent is the conjugate acid of the solvent. In aqueous solutions, the strongest acid that can exist is the hydronium ion (H₃O⁺). Since H₂SO₄ readily donates its protons to water to form H₃O⁺, it appears as a strong acid, and the differences in acidity between strong acids are masked. This means that while H₂SO₄ is a stronger acid than HSO₄⁻, their relative strengths are not fully reflected in aqueous solutions due to the leveling effect of water.
Conclusion
In summary, while sulfuric acid (H₂SO₄) doesn't possess a conjugate acid in the conventional sense, it does have a conjugate base, the bisulfate ion (HSO₄⁻). Understanding the stepwise dissociation of sulfuric acid and the properties of its conjugate base is essential for comprehending its role in various chemical processes and applications. The interplay of acid strength, solvent effects, and the leveling effect all contribute to the complex and fascinating chemistry of sulfuric acid. The focus should be placed on understanding the Brønsted-Lowry definitions correctly and the behavior of the species involved in acid-base reactions. The concept of a conjugate acid applies to bases that accept protons, not acids that donate them.
Latest Posts
Latest Posts
-
How To Solve 1 2 2
Mar 29, 2025
-
What Is 17 20 As A Decimal
Mar 29, 2025
-
What Is The Net Ionic Equation Of 2h So42
Mar 29, 2025
-
Common Factors Of 28 And 42
Mar 29, 2025
-
How To Find Area Of A Non Right Triangle
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Is The Conjugate Acid Of H2so4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
