What Is The Electron Configuration For Chlorine
listenit
Mar 26, 2025 · 6 min read
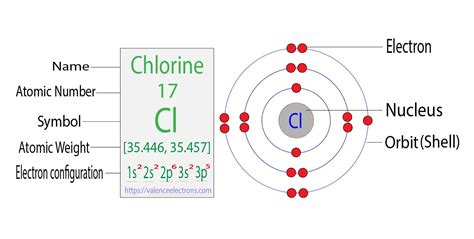
Table of Contents
What is the Electron Configuration for Chlorine? A Deep Dive into Atomic Structure
Chlorine, a vibrant yellow-green gas with a pungent odor, plays a crucial role in our daily lives, from purifying water to synthesizing essential compounds. Understanding its atomic structure, particularly its electron configuration, is key to comprehending its chemical behavior and reactivity. This comprehensive guide will delve into the electron configuration of chlorine, exploring the underlying principles of atomic structure and highlighting its implications.
Understanding Electron Configuration
Electron configuration describes the arrangement of electrons in an atom's electron shells and subshells. It dictates how an atom will interact with other atoms, forming chemical bonds and determining its chemical properties. Electrons occupy energy levels, often visualized as shells surrounding the nucleus. Each shell can hold a specific number of electrons, with the innermost shell having the lowest energy level. Within each shell are subshells, designated as s, p, d, and f, each capable of holding a different number of electrons.
The filling of these subshells follows the Aufbau principle, which states that electrons fill the lowest energy levels first. The order of filling is generally: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, and so on. However, exceptions do exist due to subtle energy level differences. Hund's rule further dictates that electrons will individually occupy orbitals within a subshell before pairing up. Finally, the Pauli Exclusion Principle ensures that no two electrons in an atom can have the same set of quantum numbers.
Determining Chlorine's Electron Configuration
Chlorine (Cl) has an atomic number of 17, meaning it has 17 protons and, in its neutral state, 17 electrons. To determine its electron configuration, we follow the Aufbau principle and fill the electron shells and subshells sequentially.
The electron configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. Let's break this down:
- 1s²: The first shell (n=1) contains only the s subshell, which can hold a maximum of two electrons. Chlorine has two electrons in this subshell.
- 2s²: The second shell (n=2) contains an s subshell (holding two electrons) and a p subshell (holding up to six electrons). Chlorine has two electrons in the 2s subshell.
- 2p⁶: The 2p subshell is completely filled with six electrons.
- 3s²: The third shell (n=3) begins with the 3s subshell, holding two electrons.
- 3p⁵: The 3p subshell has five electrons, one short of being completely filled (it can hold a maximum of six electrons).
This incomplete 3p subshell is crucial in understanding chlorine's chemical reactivity. Atoms strive for a stable electron configuration, often resembling that of a noble gas (a group 18 element with a completely filled outer electron shell). Chlorine's tendency to gain one electron to achieve a full 3p subshell (resulting in the electron configuration of Argon) drives its high reactivity.
Orbital Diagrams and Chlorine
To visualize the electron arrangement further, we can use orbital diagrams. Each subshell contains one or more orbitals, which can hold a maximum of two electrons each (with opposite spins according to the Pauli exclusion principle). For chlorine:
- 1s: Two electrons fill the single 1s orbital.
- 2s: Two electrons fill the single 2s orbital.
- 2p: The three 2p orbitals are completely filled with six electrons (two electrons in each orbital).
- 3s: Two electrons fill the single 3s orbital.
- 3p: Three of the three 3p orbitals are filled, each with one electron, and one orbital contains a pair of electrons (following Hund's rule).
Chlorine's Reactivity and its Electron Configuration
The incomplete 3p subshell in chlorine's electron configuration is directly responsible for its high reactivity. Chlorine readily forms chemical bonds by gaining one electron, achieving a stable octet (eight electrons) in its outermost shell, mimicking the electron configuration of argon. This process is known as gaining an electron. This gain of an electron leads to the formation of a negatively charged chloride ion (Cl⁻).
This strong tendency to gain an electron makes chlorine a highly effective oxidizing agent. It readily reacts with many metals and nonmetals, forming various chloride compounds. For example, the reaction between sodium (Na) and chlorine (Cl₂) produces sodium chloride (NaCl), common table salt. In this reaction, sodium loses an electron to become a positively charged ion (Na⁺), while chlorine gains an electron to become a negatively charged ion (Cl⁻). The electrostatic attraction between these oppositely charged ions forms the ionic bond in NaCl.
Beyond the Basics: Excited States and Ionization
While the ground state electron configuration discussed above is the most stable arrangement, chlorine can exist in excited states under specific conditions. In an excited state, one or more electrons jump to a higher energy level, absorbing energy in the process. This temporarily alters the electron configuration and can significantly impact the atom's reactivity. The excited state configuration is less stable and tends to revert to the ground state by releasing energy (often as light).
Ionization involves removing an electron from an atom. The energy required to remove the first electron is called the first ionization energy. Subsequent ionization energies are needed to remove further electrons. Chlorine's electron configuration helps us understand its ionization energies. The first ionization energy is relatively low compared to noble gases, reflecting the relative ease of removing the single electron from the incomplete 3p subshell. Subsequent ionization energies increase significantly as removing electrons from the more tightly bound inner shells requires substantially more energy.
Applications and Importance of Understanding Chlorine's Electron Configuration
Understanding chlorine's electron configuration is essential in various fields:
- Chemistry: Predicting reactivity, bonding behavior, and the formation of various compounds.
- Material Science: Designing materials with specific properties, such as semiconductors and catalysts, requires knowledge of the electronic structure of the constituent atoms.
- Environmental Science: Understanding chlorine's role in environmental processes, such as ozone depletion and water purification.
- Medicine: Many chlorine-containing compounds have important medicinal applications, and understanding their electronic structure is crucial for their design and synthesis.
Conclusion: The Significance of Electronic Structure
The electron configuration of chlorine, 1s²2s²2p⁶3s²3p⁵, is not just a collection of numbers and letters; it's a fundamental description of its atomic structure that dictates its chemical behavior, reactivity, and ultimately, its role in the world around us. By understanding the principles of electron configuration, we gain invaluable insights into the properties and applications of this essential element and countless others. This knowledge forms the bedrock of many scientific and technological advancements. From everyday applications like water purification to sophisticated technologies, the impact of understanding the electron configuration of elements like chlorine is undeniable.
Latest Posts
Latest Posts
-
Is Water Renewable Or Non Renewable Resource
Mar 29, 2025
-
How To Find Va And Ha
Mar 29, 2025
-
4 X 3 R 2x 5 3x 7 9x
Mar 29, 2025
-
Is Ml And Mg The Same
Mar 29, 2025
-
How To Write In Logarithmic Form
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration For Chlorine . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
