What Is The Molecular Geometry Of Pf3
listenit
Mar 18, 2025 · 6 min read
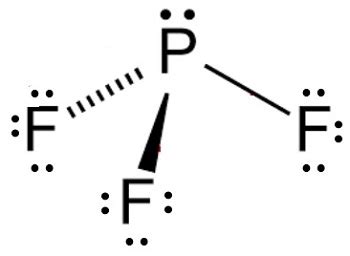
Table of Contents
What is the Molecular Geometry of PF3? A Deep Dive into Phosphorus Trifluoride
Phosphorus trifluoride (PF₃) is a fascinating molecule with significant implications in various fields, from organometallic chemistry to its potential role in the search for extraterrestrial life. Understanding its molecular geometry is crucial to grasping its reactivity and properties. This article delves into the intricacies of PF₃'s structure, explaining its shape, bond angles, and the forces that govern its configuration. We will explore the theoretical underpinnings using valence shell electron pair repulsion (VSEPR) theory and delve into the experimental evidence supporting its determined geometry.
Understanding Molecular Geometry: The VSEPR Theory
Before we analyze PF₃, let's lay the groundwork by understanding the fundamental principles of valence shell electron pair repulsion (VSEPR) theory. This theory is a cornerstone of predicting molecular geometry and is based on the premise that electron pairs, whether bonding or non-bonding (lone pairs), repel each other. This repulsion drives them to arrange themselves as far apart as possible, minimizing electron-electron interaction and thus determining the molecule's overall shape.
Key Concepts of VSEPR Theory
- Electron Domains: These encompass both bonding pairs (electrons shared between atoms) and lone pairs (electrons localized on a single atom).
- Minimizing Repulsion: The primary driving force in determining molecular geometry is the minimization of repulsion between electron domains.
- Predicting Shape: The number of electron domains dictates the basic geometry, while the presence of lone pairs modifies the actual shape of the molecule.
Determining the Molecular Geometry of PF3: A Step-by-Step Approach
Let's apply VSEPR theory to phosphorus trifluoride (PF₃).
-
Lewis Structure: First, we draw the Lewis structure of PF₃. Phosphorus (P) is the central atom with five valence electrons, and each fluorine (F) atom contributes seven valence electrons. This gives a total of 26 valence electrons. The Lewis structure shows phosphorus bonded to three fluorine atoms with three single bonds, leaving one lone pair of electrons on the phosphorus atom.
-
Electron Domains: In PF₃, there are four electron domains around the central phosphorus atom: three bonding domains (P-F bonds) and one lone pair.
-
Basic Geometry: Four electron domains predict a tetrahedral electron-pair geometry. This is the arrangement that maximizes the distance between the four electron domains.
-
Molecular Geometry: However, the molecular geometry considers only the arrangement of atoms, not the lone pairs. Since there's one lone pair, the molecular geometry of PF₃ is trigonal pyramidal. This means the three fluorine atoms are arranged around the phosphorus atom in a pyramidal shape, with the phosphorus atom at the apex and the fluorine atoms forming the base of the pyramid.
-
Bond Angles: In a perfect tetrahedron, the bond angles are 109.5°. However, the presence of the lone pair in PF₃ causes a slight distortion. The lone pair occupies more space than a bonding pair, leading to a compression of the F-P-F bond angles. The actual bond angles in PF₃ are slightly less than 109.5°, typically around 107°. This difference arises from the stronger repulsive force exerted by the lone pair compared to the bonding pairs.
Experimental Evidence Supporting the Trigonal Pyramidal Geometry
The trigonal pyramidal geometry of PF₃ isn't just a theoretical prediction; it's supported by various experimental techniques:
-
X-ray Crystallography: This powerful technique allows for the determination of the precise positions of atoms within a molecule. X-ray diffraction studies of PF₃ consistently confirm its trigonal pyramidal structure with bond angles close to 107°.
-
Microwave Spectroscopy: Analysis of the microwave spectrum of PF₃ provides valuable information about its rotational transitions. The observed spectral data is only consistent with a trigonal pyramidal structure.
-
Infrared (IR) and Raman Spectroscopy: These vibrational spectroscopic methods reveal characteristic stretching and bending vibrations of the P-F bonds. The observed vibrational frequencies are consistent with the predicted vibrational modes of a trigonal pyramidal molecule.
Comparing PF3 to Other Molecules: Understanding the Influence of Lone Pairs
To further solidify our understanding, let's compare PF₃ to other molecules with similar electronic structures:
-
Ammonia (NH₃): Similar to PF₃, NH₃ has four electron domains (three bonding and one lone pair). It also exhibits a trigonal pyramidal molecular geometry with bond angles slightly less than 109.5°. The similarity highlights the consistent effect of a lone pair on the molecular geometry.
-
Methane (CH₄): In contrast, methane has four bonding pairs and no lone pairs. Its electron-pair geometry and molecular geometry are both tetrahedral with perfect 109.5° bond angles. This comparison underscores the significant impact of lone pairs in distorting the ideal tetrahedral geometry.
-
Phosphine (PH₃): While similar to PF₃ in having a central phosphorus atom and three attached groups, phosphine has three hydrogen atoms instead of fluorine atoms. The electronegativity difference affects the bond angles slightly, but the overall trigonal pyramidal shape remains consistent.
The Importance of Molecular Geometry in PF3's Reactivity and Properties
The trigonal pyramidal geometry of PF₃ significantly influences its chemical behavior and properties:
-
Polarity: Due to the asymmetrical distribution of electron density, PF₃ is a polar molecule. The electronegativity difference between phosphorus and fluorine leads to a net dipole moment, impacting its interactions with other molecules and its solubility in various solvents.
-
Reactivity: The presence of the lone pair on the phosphorus atom makes PF₃ a Lewis base. It can donate this lone pair to electron-deficient species, forming coordinate covalent bonds. This reactivity is fundamental in its applications in organometallic chemistry and catalysis.
-
Spectroscopic Properties: The molecular geometry dictates the vibrational and rotational modes of PF₃, which are observed in its IR and microwave spectra. This allows for its identification and characterization.
-
Biological Implications: While not directly used biologically, PF3's structure serves as a model for understanding the behavior of similar molecules in biological systems. Moreover, its potential as a biomarker in the search for extraterrestrial life stems from its unique infrared signature related to its structure.
Conclusion: A Comprehensive Understanding of PF3's Molecular Geometry
The molecular geometry of phosphorus trifluoride (PF₃) is a critical aspect of understanding its properties and reactivity. Through the application of VSEPR theory and the corroboration of experimental data, we have established its trigonal pyramidal structure with bond angles slightly less than the ideal tetrahedral angle. This geometry, dictated by the presence of a lone pair on the phosphorus atom, directly influences PF₃'s polarity, reactivity as a Lewis base, spectroscopic characteristics, and potential implications in various scientific fields. This in-depth understanding highlights the power of theoretical models and experimental techniques in revealing the intricacies of molecular structures and their consequences. Further research into its behavior and potential applications promises to yield valuable insights in chemistry and related disciplines.
Latest Posts
Latest Posts
-
Atoms Of The Same Element Have The Same Number Of
Mar 19, 2025
-
Can A Element Be Broken Down
Mar 19, 2025
-
Calculating The Ph At The Equivalence Point
Mar 19, 2025
-
What Is The Equivalent Fraction For 3 4
Mar 19, 2025
-
What Does A High Specific Heat Capacity Mean
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molecular Geometry Of Pf3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
