What Does A High Specific Heat Capacity Mean
listenit
Mar 19, 2025 · 6 min read
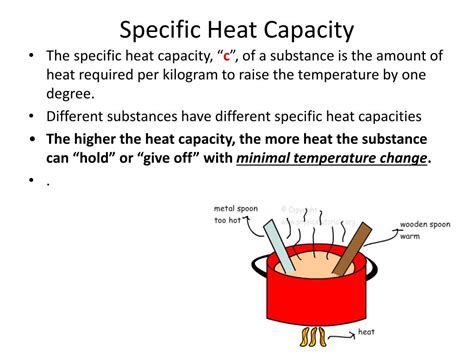
Table of Contents
What Does a High Specific Heat Capacity Mean?
Specific heat capacity is a fundamental concept in physics and chemistry, crucial for understanding how substances respond to changes in temperature. This seemingly simple property has far-reaching implications, influencing everything from the design of engine cooling systems to the regulation of Earth's climate. So, what does a high specific heat capacity actually mean? Let's dive deep into this fascinating topic.
Understanding Specific Heat Capacity: The Basics
Specific heat capacity (often abbreviated as c) is the amount of heat required to raise the temperature of one kilogram (or one gram, depending on the units used) of a substance by one degree Celsius (or one Kelvin). It's a measure of a substance's resistance to temperature change. In simpler terms, it tells us how much energy we need to input to increase its temperature, or conversely, how much energy is released when its temperature decreases.
The units for specific heat capacity are typically J/(kg·K) or J/(g·°C), representing Joules per kilogram-Kelvin or Joules per gram-degree Celsius. These units highlight the relationship between heat energy (Joules) and the resulting temperature change.
The Significance of "Specific"
The word "specific" is key here. It emphasizes that this property is specific to each substance. Different materials have different capacities for absorbing and storing heat. This difference arises from variations in the molecular structure, bonding, and interactions within the material.
What a High Specific Heat Capacity Means
A high specific heat capacity means a substance requires a significant amount of heat energy to raise its temperature by a given amount. Conversely, it also means that it releases a significant amount of heat energy when its temperature decreases. This inherent resistance to temperature change is the defining characteristic.
Think of it like this: Imagine you have two pots of equal mass, one filled with water and the other with oil. If you apply the same amount of heat to both pots, the water's temperature will increase much more slowly than the oil's. This is because water has a significantly higher specific heat capacity than oil.
Why Do Some Substances Have High Specific Heat Capacities?
The high specific heat capacity of a substance is directly linked to the way its molecules interact with each other and with the energy they absorb. Several factors contribute:
-
Hydrogen Bonding: Water, famously known for its high specific heat capacity, owes this to the strong hydrogen bonds between its molecules. These bonds require a significant amount of energy to break, thus increasing the heat needed to raise the temperature. This is crucial for life on Earth, as it helps moderate temperature fluctuations in aquatic environments and within living organisms.
-
Molecular Complexity: More complex molecules with many degrees of freedom (ways to store energy) generally have higher specific heat capacities. This is because the energy absorbed can be distributed among various vibrational, rotational, and translational modes within the molecule, making it harder to increase the overall kinetic energy (and thus temperature) of the system.
-
Intermolecular Forces: Strong intermolecular forces (like dipole-dipole interactions or London dispersion forces) require considerable energy to overcome, thereby increasing the specific heat capacity.
Real-World Examples of High Specific Heat Capacity Materials
Several materials exhibit notably high specific heat capacities, each with unique applications:
-
Water: Water's exceptionally high specific heat capacity (4186 J/(kg·K)) makes it an excellent coolant and temperature regulator. It's used in car engines, industrial processes, and even in our own bodies to maintain stable temperatures.
-
Sand: While not as high as water's, sand still possesses a relatively high specific heat capacity compared to many other solids. This property plays a role in regulating coastal temperatures.
-
Liquid Metals (e.g., Mercury, Sodium): Certain liquid metals, though not possessing the highest specific heat capacities, still hold more than some other common materials. Their high thermal conductivity and specific heat makes them effective coolants in applications like nuclear reactors.
-
Concrete and Soil: These materials also have relatively high specific heat capacities, contributing to the thermal inertia of buildings and land, moderating temperature fluctuations.
Applications of High Specific Heat Capacity
The characteristic of high specific heat capacity has numerous practical applications across various fields:
1. Climate Regulation:
Water's high specific heat capacity is crucial for regulating Earth's climate. Large bodies of water absorb immense amounts of solar radiation without experiencing drastic temperature increases, acting as buffers against extreme temperature swings. This moderates coastal climates and prevents rapid temperature fluctuations globally.
2. Engine Cooling:
The high specific heat capacity of water makes it an ideal coolant for internal combustion engines. Water circulates through the engine, absorbing heat generated by combustion, preventing overheating. This allows for efficient and safe engine operation.
3. Industrial Processes:
Many industrial processes utilize substances with high specific heat capacities to control temperature. Heat transfer fluids in chemical reactors and power plants often rely on materials with this property for efficient temperature control and prevention of thermal shock.
4. Building Design:
In architecture and construction, materials with high specific heat capacities can contribute to energy efficiency. They can act as thermal mass, absorbing heat during the day and releasing it at night, reducing the need for heating and cooling systems.
5. Food Processing:
In food processing, the specific heat capacity plays a role in controlling temperatures during cooking, preserving, and packaging processes.
Low Specific Heat Capacity: The Opposite Side of the Coin
Understanding high specific heat capacity also necessitates understanding its opposite: low specific heat capacity. Substances with low specific heat capacities readily change temperature with relatively small amounts of heat exchange. This property is utilized in applications where rapid heating or cooling is required. Examples include:
-
Metals (e.g., Aluminum, Copper): Metals generally have relatively low specific heat capacities, making them suitable for cookware and heat exchangers where rapid heating is needed.
-
Gases: Gases typically have much lower specific heat capacities than liquids or solids because their molecules are farther apart, resulting in weaker intermolecular interactions.
Conclusion: The Importance of Specific Heat Capacity
Specific heat capacity is a crucial property influencing numerous aspects of our world, from the climate we live in to the technologies we use. Understanding what a high specific heat capacity means—that a substance resists changes in temperature—opens a door to appreciating its vast significance in both natural phenomena and technological applications. The ability of a material to absorb and release heat energy plays a vital role in various fields, emphasizing the importance of this fundamental concept in physics and chemistry. Further exploration of this property can lead to innovations in energy efficiency, materials science, and many other areas.
Latest Posts
Latest Posts
-
How Many Inches Is 1 8 Of A Yard
Mar 19, 2025
-
Find The Limit Of The Sequence
Mar 19, 2025
-
What Is The Formula For Calcium Fluoride
Mar 19, 2025
-
Is Wood Burning A Physical Or Chemical Change
Mar 19, 2025
-
Can A Trapezoid Be A Square
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Does A High Specific Heat Capacity Mean . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
