Atoms Of The Same Element Have The Same Number Of
listenit
Mar 19, 2025 · 6 min read
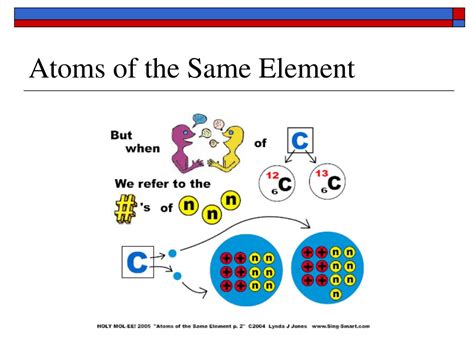
Table of Contents
Atoms of the Same Element Have the Same Number of Protons: A Deep Dive into Atomic Structure
The fundamental building blocks of matter, atoms, are incredibly tiny yet incredibly complex. Understanding their structure is key to understanding the properties of all substances, from the air we breathe to the stars in the sky. A core principle governing atomic structure is that atoms of the same element have the same number of protons. This seemingly simple statement underpins a vast amount of chemistry and physics. This article will delve deep into this principle, exploring its implications and connections to other atomic properties.
What Defines an Element?
Before diving into the specifics of proton number, let's clarify what constitutes an element. An element is a pure substance consisting entirely of atoms with the same number of protons in their nuclei. This number, known as the atomic number, is unique to each element and determines its position on the periodic table. For example, all hydrogen atoms have one proton, oxygen atoms have eight, and gold atoms have 79. This proton count is what fundamentally distinguishes one element from another. No two elements share the same atomic number.
The Role of the Nucleus
The atom's nucleus is its central core, comprising almost all of the atom's mass. It contains two types of subatomic particles: protons and neutrons. Protons carry a positive electrical charge, while neutrons are electrically neutral. The number of protons defines the element, but the number of neutrons can vary, leading to isotopes.
Isotopes: Variations on a Theme
Isotopes are atoms of the same element that have the same number of protons but a different number of neutrons. This difference in neutron number affects the atom's mass but not its chemical properties. For example, carbon-12 (⁶C) has six protons and six neutrons, while carbon-14 (¹⁴C) has six protons and eight neutrons. Both are carbon atoms because they both have six protons, but their different neutron counts give them slightly different masses and radioactive properties. Carbon-14 is radioactive, making it useful in dating organic materials.
The Significance of Atomic Number
The atomic number, representing the number of protons, is crucial for several reasons:
-
Chemical Identity: As previously stated, the atomic number uniquely identifies an element. It dictates the element's position on the periodic table and its chemical behavior. Elements with similar atomic numbers often exhibit similar chemical properties due to the arrangement of their electrons.
-
Electron Configuration: The number of protons directly influences the number of electrons an atom possesses in a neutral state. Atoms are electrically neutral; therefore, the number of electrons equals the number of protons. This electron configuration determines the atom's reactivity and how it interacts with other atoms to form molecules and compounds.
-
Periodic Trends: The periodic table organizes elements based on their atomic numbers and recurring chemical properties. This arrangement reveals trends in properties like electronegativity, ionization energy, and atomic radius, all directly linked to the number of protons and the resulting electron configuration.
Electrons and Chemical Behavior
While the number of protons defines the element, it's the electrons that are primarily involved in chemical reactions. Electrons occupy specific energy levels or shells surrounding the nucleus. The outermost electrons, called valence electrons, are particularly important in determining an element's reactivity. Atoms tend to gain, lose, or share valence electrons to achieve a stable electron configuration, typically a full outermost shell. This drive for stability is the foundation of chemical bonding.
Types of Chemical Bonds
The way atoms interact to achieve stable electron configurations leads to different types of chemical bonds:
-
Ionic Bonds: Involve the transfer of electrons from one atom to another, creating ions with opposite charges that attract each other. This usually happens between atoms with vastly different electronegativities.
-
Covalent Bonds: Involve the sharing of electrons between atoms, creating a more stable electron configuration for both. This is common between atoms with similar electronegativities.
-
Metallic Bonds: Occur in metals, where electrons are delocalized and shared amongst a lattice of positively charged metal ions. This allows for the characteristic properties of metals, such as electrical and thermal conductivity.
Beyond the Basics: Exploring Isotopes in Depth
Isotopes, while having the same atomic number, exhibit differences in their physical properties due to their varying neutron counts:
-
Mass Number: The mass number is the total number of protons and neutrons in an atom's nucleus. Isotopes of the same element have different mass numbers.
-
Nuclear Stability: The ratio of protons to neutrons in the nucleus affects its stability. Some isotopes are stable, while others are radioactive, meaning their nuclei spontaneously decay, emitting particles and energy. This radioactive decay has many applications in medicine, research, and dating techniques.
-
Nuclear Reactions: Isotopes play a crucial role in nuclear reactions, including nuclear fission and fusion. These processes involve changes to the atom's nucleus, often resulting in the formation of different isotopes or elements.
-
Applications of Isotopes: Radioactive isotopes find numerous applications:
- Medical Imaging: Techniques like PET (positron emission tomography) scans use radioactive isotopes to diagnose diseases.
- Cancer Therapy: Radioactive isotopes are used in radiotherapy to target and destroy cancer cells.
- Carbon Dating: The radioactive isotope carbon-14 is used to date organic materials.
The Periodic Table and Atomic Number
The periodic table's organization is directly related to atomic number. Elements are arranged in order of increasing atomic number, revealing recurring patterns in their properties. This periodic repetition, or periodicity, is due to the regular filling of electron shells. Elements in the same group (vertical column) have similar chemical properties because they have the same number of valence electrons.
Conclusion: A Foundation for Understanding Matter
The principle that atoms of the same element have the same number of protons is a fundamental concept in chemistry and physics. It provides the basis for understanding the organization of the periodic table, the behavior of elements, and the formation of chemical bonds. The concept extends to encompass isotopes, nuclear reactions, and various applications in science and technology. By appreciating this fundamental truth, we can gain a deeper understanding of the nature of matter and the intricate world of atoms and their interactions. From the smallest molecules to the largest stars, the number of protons remains a defining characteristic of an element, a cornerstone of our understanding of the universe. Further exploration into atomic structure and nuclear physics reveals even more complexities and fascinating applications of this principle. The constant advancement in scientific understanding continues to build upon this foundational knowledge, further emphasizing the importance of this core principle in the study of matter. The seemingly simple statement – atoms of the same element have the same number of protons – unlocks a universe of scientific understanding.
Latest Posts
Latest Posts
-
How Many Ounces Is 500 Ml Of Water
Mar 19, 2025
-
Revolutions Per Second To Radians Per Second
Mar 19, 2025
-
Whats The Square Root Of 60
Mar 19, 2025
-
What Are The Subunits Of Dna
Mar 19, 2025
-
How Many Inches Is 1 8 Of A Yard
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Atoms Of The Same Element Have The Same Number Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
