Calculating The Ph At The Equivalence Point
listenit
Mar 19, 2025 · 6 min read
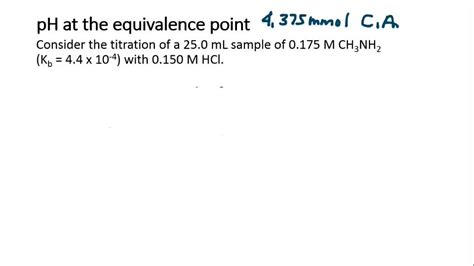
Table of Contents
Calculating the pH at the Equivalence Point: A Comprehensive Guide
Determining the pH at the equivalence point of a titration is crucial for understanding acid-base reactions and performing accurate quantitative analyses. This point signifies the complete neutralization of an acid by a base, or vice versa. However, calculating the exact pH isn't always straightforward, as it depends on the strengths of the acid and base involved. This comprehensive guide will explore various scenarios, providing you with the tools and knowledge to accurately calculate the pH at the equivalence point in different titrations.
Understanding the Equivalence Point
The equivalence point in a titration is the point at which the moles of acid are stoichiometrically equal to the moles of base added (or vice versa). It's a theoretical point determined through calculations. The endpoint, on the other hand, is the point observed experimentally using an indicator. Ideally, the endpoint and equivalence point should coincide, but slight discrepancies can occur.
Calculating pH at the Equivalence Point: Different Scenarios
The method for calculating the pH at the equivalence point varies depending on the nature of the acid and base involved:
1. Strong Acid - Strong Base Titration
This is the simplest case. The equivalence point of a strong acid-strong base titration always results in a neutral solution with a pH of 7. This is because the reaction produces only water and a neutral salt:
Example: HCl (strong acid) + NaOH (strong base) → NaCl (neutral salt) + H₂O
Since both the acid and base are completely dissociated, the resulting solution contains only the conjugate ions, which do not significantly affect the pH.
2. Weak Acid - Strong Base Titration
This scenario is more complex. At the equivalence point, the weak acid (HA) has been completely neutralized by the strong base (OH⁻), forming the conjugate base (A⁻). The conjugate base will react with water, leading to hydrolysis and a basic pH.
Calculating the pH:
-
Determine the concentration of the conjugate base: This requires knowing the initial moles of weak acid and the volume of strong base added at the equivalence point. The moles of conjugate base will be equal to the initial moles of weak acid. The new total volume is the sum of the initial acid volume and the base volume added at the equivalence point.
-
Set up an ICE table: This table helps track the changes in concentration of the species involved in the hydrolysis reaction:
Species Initial (M) Change (M) Equilibrium (M) A⁻ [A⁻]ᵢ -x [A⁻]ᵢ - x HA 0 +x x OH⁻ 0 +x x -
Write the Kb expression: Kb = Kw/Ka, where Kw is the ion product of water (1.0 x 10⁻¹⁴ at 25°C) and Ka is the acid dissociation constant of the weak acid.
-
Solve for x: Substitute the equilibrium concentrations from the ICE table into the Kb expression and solve for x, which represents the hydroxide ion concentration ([OH⁻]).
-
Calculate pOH: pOH = -log[OH⁻]
-
Calculate pH: pH = 14 - pOH
Example: Consider the titration of 0.1 M acetic acid (Ka = 1.8 x 10⁻⁵) with 0.1 M NaOH. At the equivalence point, all acetic acid is converted to acetate. The concentration of acetate can be calculated, and the subsequent hydrolysis will determine the pH. The pH will be greater than 7.
3. Weak Base - Strong Acid Titration
Similar to the previous case, at the equivalence point, the weak base (B) is completely neutralized by the strong acid (H⁺), forming the conjugate acid (BH⁺). The conjugate acid will react with water, leading to hydrolysis and an acidic pH.
Calculating the pH:
The process is analogous to the weak acid - strong base titration. The key difference is that you'll use the Ka expression for the conjugate acid (Ka = Kw/Kb) and solve for [H⁺] to determine the pH. The pH will be less than 7.
4. Polyprotic Acid Titration
Polyprotic acids can donate more than one proton. Their titration curves exhibit multiple equivalence points, each requiring a separate pH calculation. Each equivalence point corresponds to the complete neutralization of one proton.
Calculating the pH:
Each equivalence point will generate a different species (e.g., H₂A, HA⁻, A²⁻ for a diprotic acid). The pH at each equivalence point depends on the equilibrium of the remaining species with water. You'll need to consider the relevant Ka values for each dissociation step.
5. Weak Acid - Weak Base Titration
This is the most challenging case. The pH at the equivalence point is determined by the relative strengths of the weak acid and weak base, and the resulting salt formed. A simple formula doesn't exist, and iterative numerical methods are usually necessary to solve the resulting equilibrium equations.
Factors Affecting pH at the Equivalence Point
Several factors can influence the pH at the equivalence point:
-
Temperature: Kw changes with temperature, affecting the pH calculations, particularly for weak acid-strong base or weak base-strong acid titrations.
-
Ionic Strength: High ionic strength can affect the activity coefficients of the ions, leading to deviations from ideal behavior.
-
Concentration: The concentration of the acid and base can influence the pH, especially in cases involving weak acids or bases.
-
Presence of Other Ions: The presence of other ions in the solution can affect the pH through common ion effects or complex formation.
Using the Henderson-Hasselbalch Equation (Approximation)
While not perfectly accurate for all equivalence points, the Henderson-Hasselbalch equation can provide a reasonable approximation, particularly for titrations close to the equivalence point:
pH = pKa + log([A⁻]/[HA])
This equation applies mainly to buffer regions, which exist before the equivalence point in weak acid-strong base titrations and after the equivalence point in weak base-strong acid titrations. At the equivalence point itself, this equation becomes less accurate.
Importance of Accurate pH Calculation
Precise calculation of the pH at the equivalence point is critical for:
-
Quantitative analysis: Titration is a fundamental technique in analytical chemistry for determining the concentration of unknown solutions.
-
Understanding acid-base chemistry: Calculating the pH helps us understand the equilibrium properties of acids and bases and their reactions.
-
Optimizing chemical processes: Many chemical processes are pH-sensitive, and precise pH control is essential for efficiency and yield.
-
Environmental monitoring: Acid-base titrations are used to monitor water quality and other environmental parameters.
Conclusion
Calculating the pH at the equivalence point involves understanding the acid-base properties of the reactants and the equilibrium of the resulting species. The approach varies depending on the strengths of the acid and base involved. While simple calculations apply to strong acid-strong base titrations, calculating the pH for weak acid-strong base or weak base-strong acid titrations requires a deeper understanding of hydrolysis and equilibrium constants. For polyprotic acids and weak acid-weak base titrations, more advanced techniques may be needed. However, with careful application of the appropriate methods, accurate determination of the pH at the equivalence point is achievable. This knowledge is crucial for various applications in chemistry and related fields.
Latest Posts
Latest Posts
-
Find The Limit Of The Sequence
Mar 19, 2025
-
What Is The Formula For Calcium Fluoride
Mar 19, 2025
-
Is Wood Burning A Physical Or Chemical Change
Mar 19, 2025
-
Can A Trapezoid Be A Square
Mar 19, 2025
-
How Much Is A Quarter Pound In Ounces
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Calculating The Ph At The Equivalence Point . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
