What Is The Horizontal Rows On The Periodic Table Called
listenit
Mar 15, 2025 · 6 min read
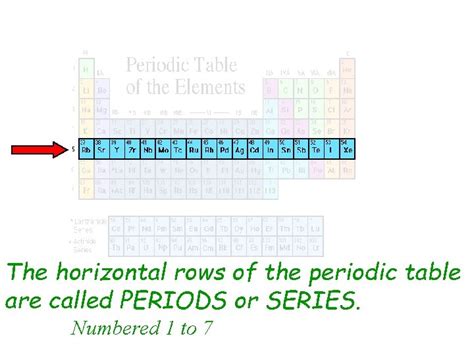
Table of Contents
What are the Horizontal Rows on the Periodic Table Called? A Deep Dive into Periods and Their Significance
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic number and recurring chemical properties. While many are familiar with the vertical columns, known as groups or families, understanding the horizontal rows, called periods, is equally crucial to grasping the fundamental principles of chemistry. This comprehensive guide will delve into what periods are, how they are organized, the trends they exhibit, and their significance in predicting and understanding the behavior of elements.
Understanding Periods: A Horizontal Journey Through Atomic Structure
The horizontal rows in the periodic table are known as periods. Each period represents a principal energy level or electron shell. As we move across a period from left to right, the atomic number increases, meaning the number of protons and electrons in the atom also increases. This addition of electrons happens within the same principal energy level.
The Significance of Electron Shells
The arrangement of electrons in these shells dictates the chemical properties of an element. Elements within the same period have electrons filling the same outermost electron shell (valence shell). This shared characteristic significantly influences their reactivity and other chemical behaviors.
Key takeaway: The number of the period corresponds to the highest principal quantum number (n) of the electrons in an element's ground state electron configuration. For example, elements in period 1 have electrons only in the n=1 shell, elements in period 2 have electrons in n=1 and n=2 shells, and so on.
The Structure of Periods: A Closer Look
Let's explore the structure of periods in more detail. Each period starts with an alkali metal (except for period 1, which begins with hydrogen) and ends with a noble gas. The number of elements in each period varies, reflecting the increasing complexity of electron shells as we move down the periodic table.
Period 1: The Shortest Period
Period 1 is unique, containing only two elements: hydrogen (H) and helium (He). Both elements have electrons filling the first principal energy level (n=1), which can only hold a maximum of two electrons. This short period represents the simplest atomic structure.
Period 2 and 3: The Short Periods
Periods 2 and 3 are considered short periods, each containing eight elements. These elements have their valence electrons filling the second (n=2) and third (n=3) principal energy levels respectively. The filling of these energy levels follows the Aufbau principle and Hund's rule, leading to predictable electronic configurations and chemical properties.
Period 4 and 5: The Long Periods
Periods 4 and 5 are long periods, each containing 18 elements. This increase in the number of elements is due to the introduction of d-orbitals. These orbitals can hold up to 10 electrons, resulting in a significant expansion of the period length.
Period 6 and 7: The Longest Periods
Periods 6 and 7 are the longest periods, with 32 elements each. The significant length is attributed to the filling of f-orbitals, which can hold up to 14 electrons. These f-orbitals are responsible for the lanthanides and actinides, which are often placed separately at the bottom of the periodic table for ease of presentation.
Periodic Trends Across Periods: Observing the Patterns
As we move across a period from left to right, several key trends in the properties of elements emerge. Understanding these trends allows us to predict the behavior of elements and their reactivity.
Atomic Radius: A Gradual Decrease
Atomic radius, the distance between the nucleus and the outermost electron, generally decreases across a period. This is because the number of protons in the nucleus increases, leading to a stronger attraction for the electrons, pulling them closer to the nucleus.
Ionization Energy: A General Increase
Ionization energy, the energy required to remove an electron from an atom, generally increases across a period. This is due to the increasing nuclear charge and decreasing atomic radius, making it more difficult to remove an electron from an atom.
Electronegativity: An Increasing Tendency
Electronegativity, the ability of an atom to attract electrons towards itself in a chemical bond, generally increases across a period. This is because of the increasing nuclear charge and decreasing atomic radius, making it easier for the atom to attract electrons.
Metallic Character: A Transition from Metal to Nonmetal
Metallic character, the tendency of an element to lose electrons and form positive ions, generally decreases across a period. Metals are found on the left side of the periodic table, while nonmetals are on the right. This transition reflects the increasing electronegativity and ionization energy across the period.
The Significance of Periods in Predicting Chemical Behavior
The organization of elements into periods provides a powerful tool for predicting chemical behavior. The similar electron configurations of elements within the same period lead to similar chemical properties. For example, elements in the same period often exhibit similar reactivity patterns. This understanding is essential in various chemical applications, including:
- Predicting chemical reactions: By knowing the period of an element, we can estimate its reactivity and potential reactions with other elements.
- Understanding bonding characteristics: The location of an element within a period helps us predict the type of bonds it will form (ionic, covalent, metallic).
- Designing new materials: The knowledge of periodic trends enables the design and synthesis of materials with specific properties.
Beyond the Basics: Deeper Implications of Periodicity
The periodic table's organization, particularly the periods, is not merely a classification system; it reflects fundamental principles of atomic structure and quantum mechanics. The repeating patterns in the properties of elements stem from the periodic filling of electron orbitals. This periodicity underscores the elegant mathematical relationships governing the behavior of matter at its most fundamental level.
Applications in Various Scientific Fields
The concepts of periods and periodic trends find applications far beyond the realm of basic chemistry. These include:
- Materials science: Understanding periodic trends helps in the development of novel materials with tailored properties.
- Biochemistry: The behavior of biologically important elements is directly related to their position within periods.
- Nuclear chemistry: The stability and radioactivity of elements are often correlated with their period and group.
- Environmental chemistry: The reactivity and environmental impact of elements are tied to their periodic properties.
Conclusion: The Unfolding Story of Periods
The horizontal rows, or periods, on the periodic table are far more than a simple arrangement; they represent a profound insight into the fundamental structure of matter and the predictable behavior of elements. By understanding the principles of electron shell filling and the periodic trends, we can unlock a deeper appreciation for the intricate relationships between atoms and their macroscopic properties. The periodic table, with its periods and groups, remains a powerful tool and an ongoing source of scientific discovery and innovation. Its enduring utility lies in its ability to simplify and systematize the complexity of the chemical world, making it accessible for learning and exploration. From understanding basic chemical reactions to designing cutting-edge materials, the periods on the periodic table serve as an invaluable framework for scientific progress.
Latest Posts
Latest Posts
-
X 4 2x 2 8 0
Mar 15, 2025
-
Whats The Square Root Of 28
Mar 15, 2025
-
Where Is The Most Mass Of An Atom Found
Mar 15, 2025
-
What Is The Lowest Common Factor Of 12 And 15
Mar 15, 2025
-
Give The Formula For Plumbous Nitrate
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about What Is The Horizontal Rows On The Periodic Table Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
