Where Is The Most Mass Of An Atom Found
listenit
Mar 15, 2025 · 5 min read
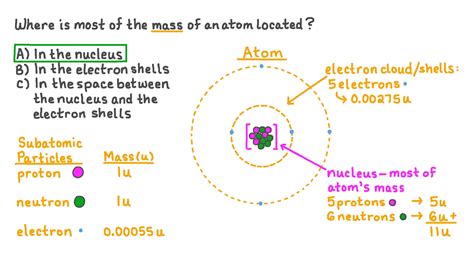
Table of Contents
Where is the Most Mass of an Atom Found? Delving into the Atomic Nucleus
The atom, the fundamental building block of matter, is a fascinating microcosm of complexity. Understanding its structure is key to grasping the properties of everything around us. A common question that arises is: where is the majority of an atom's mass located? The simple, and accurate, answer is: the nucleus. But to fully appreciate this answer, we need to delve deeper into the components of an atom and their relative contributions to its overall mass.
The Atomic Structure: A Brief Overview
Before we pinpoint the location of an atom's mass, let's refresh our understanding of atomic structure. Atoms consist of three primary subatomic particles:
- Protons: Positively charged particles residing within the atom's nucleus.
- Neutrons: Neutral particles (no charge) also located within the nucleus.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels.
The arrangement of these particles determines an atom's properties and how it interacts with other atoms. Crucially, the mass of these particles differs significantly.
Mass of Subatomic Particles
The mass of a proton is approximately 1.6726 × 10⁻²⁷ kg. The mass of a neutron is very similar, at about 1.6749 × 10⁻²⁷ kg. In contrast, the mass of an electron is significantly smaller, at approximately 9.1094 × 10⁻³¹ kg. This difference is crucial when considering where the bulk of an atom's mass resides. Notice that protons and neutrons are nearly 2000 times more massive than an electron.
The Nucleus: The Heart of Atomic Mass
Given the mass disparity between protons, neutrons, and electrons, it's clear that the vast majority of an atom's mass is concentrated in the nucleus. This tiny, dense region at the atom's center houses both protons and neutrons, collectively known as nucleons. The electrons, despite their charge's significance in chemical reactions, contribute negligibly to the overall mass.
Calculating Atomic Mass
An atom's mass number (A) represents the total number of protons and neutrons in its nucleus. This number directly reflects the nucleus's contribution to the atom's overall mass. The atomic number (Z), on the other hand, represents the number of protons, which defines the element. The number of neutrons (N) is given by A - Z.
For example, a carbon-12 atom (¹²C) has an atomic number of 6 (6 protons) and a mass number of 12 (6 protons + 6 neutrons). Almost all of its mass (12 atomic mass units or amu) is concentrated within the nucleus, with the electrons contributing only a tiny fraction.
The Negligible Mass of Electrons
While electrons play a vital role in chemical bonding and determining an atom's reactivity, their minuscule mass compared to protons and neutrons makes their contribution to the total atomic mass essentially negligible. Their presence is critical for the atom's overall stability and behavior, but in terms of mass, they are inconsequential.
Electron Cloud vs. Nucleus: A Size Comparison
The atom's structure is also characterized by a vast difference in scale between the nucleus and the electron cloud. The nucleus occupies an incredibly small volume compared to the overall size of the atom. Imagine a stadium; the nucleus would be like a marble at the center, while the electrons would be like tiny particles circling the entire stadium. This immense space occupied by the electron cloud further emphasizes the concentration of mass within the minuscule nucleus.
Isotopes and Atomic Mass: A Deeper Dive
Isotopes are atoms of the same element with the same number of protons but a different number of neutrons. This means they have the same atomic number (Z) but different mass numbers (A). Isotopes of an element exhibit similar chemical properties but can have different physical properties due to their varying mass.
The atomic mass (or atomic weight) listed on the periodic table is a weighted average of the masses of all naturally occurring isotopes of an element, considering their relative abundance. This weighted average reflects the typical mass of an atom of that element, which is predominantly determined by the mass of the nucleus.
Examples of Isotopes and Mass Distribution
Consider the element chlorine (Cl). Chlorine has two main isotopes: ³⁵Cl and ³⁷Cl. ³⁵Cl accounts for about 75% of naturally occurring chlorine, while ³⁷Cl makes up the remaining 25%. The atomic mass of chlorine on the periodic table is approximately 35.45 amu, which is a weighted average reflecting the mass contribution of both isotopes. Again, the majority of this mass resides in the nucleus of each chlorine atom.
Beyond the Atom: Mass in Molecules and Compounds
The principle of mass concentration in the nucleus extends to molecules and compounds. The mass of a molecule is essentially the sum of the atomic masses of its constituent atoms. Since the mass of each atom is largely determined by its nucleus, the overall mass of a molecule is also primarily dictated by the nuclei of its atoms.
Conclusion: The Nucleus Reigns Supreme
In conclusion, the vast majority—over 99.9%—of an atom's mass is concentrated within its nucleus. While electrons are crucial for an atom's chemical behavior and overall stability, their minuscule mass compared to protons and neutrons makes their contribution to the total atomic mass insignificant. Understanding this fundamental aspect of atomic structure is crucial for comprehending the properties of matter at both the atomic and macroscopic levels. The nucleus, that tiny, dense heart of the atom, holds the key to understanding the mass of everything around us.
Latest Posts
Latest Posts
-
60 Is 40 Of What Number
Mar 15, 2025
-
114 Kg Is How Many Pounds
Mar 15, 2025
-
Proof Of Sin 2x Cos 2x 1
Mar 15, 2025
-
How Many Liters Are In 1500 Milliliters
Mar 15, 2025
-
How Many Ounces In A 5th
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Where Is The Most Mass Of An Atom Found . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
