What Is The Equivalence Point In A Titration
listenit
Mar 22, 2025 · 8 min read
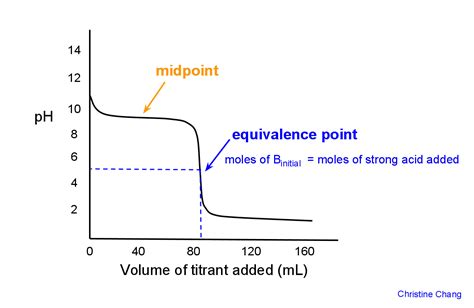
Table of Contents
What is the Equivalence Point in a Titration? A Comprehensive Guide
Titration, a cornerstone technique in analytical chemistry, allows for the precise determination of an unknown concentration of a substance (analyte) by reacting it with a solution of known concentration (titrant). Understanding the equivalence point is crucial for accurate and reliable results in any titration experiment. This comprehensive guide delves deep into the concept of the equivalence point, explaining its significance, how it differs from the endpoint, and the factors influencing its determination.
Understanding the Basics: Titration and its Components
Before we dive into the equivalence point, let's establish a foundational understanding of titration. Titration involves the gradual addition of a titrant to a solution containing the analyte until the reaction between them is complete. This reaction is typically an acid-base neutralization, a redox reaction, or a complexation reaction. Key components of a titration include:
- Analyte: The substance with an unknown concentration that we are trying to determine.
- Titrant: The solution of known concentration that is added to the analyte.
- Indicator: A substance that changes color near the equivalence point, signaling the endpoint of the titration. The choice of indicator depends on the specific titration being performed.
- Buret: A graduated glass tube used to deliver the titrant precisely.
- Erlenmeyer Flask: A conical flask that holds the analyte solution.
Defining the Equivalence Point: The Heart of the Titration
The equivalence point is the theoretical point in a titration where the amount of titrant added is stoichiometrically equivalent to the amount of analyte present. In simpler terms, it's the point at which the moles of titrant added have completely reacted with the moles of analyte in the solution. At this point, the reaction between the titrant and the analyte is complete. It's a crucial point because it represents the exact moment when the analyte has been fully neutralized or reacted with the titrant.
Let's illustrate with an example: Consider a titration of a strong acid (HCl) with a strong base (NaOH). The balanced chemical equation for this reaction is:
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
The equivalence point is reached when the moles of HCl are equal to the moles of NaOH added. This is determined using the following equation:
Moles of HCl = Molarity of HCl × Volume of HCl
Moles of NaOH = Molarity of NaOH × Volume of NaOH
At the equivalence point: Moles of HCl = Moles of NaOH
This relationship allows us to calculate the unknown concentration of either the acid or the base if we know the concentration of the other.
Differentiating Equivalence Point and Endpoint: A Subtle but Crucial Distinction
While both the equivalence point and the endpoint mark the end of a titration, they are not the same. The endpoint is the point at which the indicator changes color, visually signaling the completion of the reaction. The endpoint is an observable phenomenon, whereas the equivalence point is a theoretical point that we aim to reach.
The difference between the equivalence point and the endpoint is called the titration error. This error arises because the indicator's color change doesn't occur precisely at the equivalence point. The indicator’s color change is typically observed in a narrow range around the equivalence point, but this range represents a small error in the calculations. This error can be minimized by choosing an appropriate indicator that changes color very close to the equivalence point of the reaction.
Careful selection of the indicator is crucial to minimize the titration error. The indicator's pH range should encompass the pH at the equivalence point. For example, phenolphthalein is commonly used for strong acid-strong base titrations because its color change occurs around pH 8-10, which is close to the equivalence point of these titrations (pH 7). However, in weak acid-strong base titrations or weak base-strong acid titrations, the equivalence point pH is not 7, and thus, a different indicator should be employed.
Factors Affecting the Equivalence Point Determination
Several factors can influence the accuracy of equivalence point determination:
- Temperature: Temperature affects the solubility of reactants and the equilibrium constant of the reaction, potentially shifting the equivalence point. Maintaining a consistent temperature throughout the titration is vital.
- Solvent: The solvent used can also influence the equilibrium constant of the reaction and therefore, the equivalence point. The use of different solvents such as water, ethanol, or mixtures of water and ethanol will impact the reaction and result in a different equivalence point.
- Indicator Choice: As mentioned earlier, the indicator's pH range must be carefully considered to minimize the difference between the equivalence point and the endpoint. The wrong indicator choice can lead to significant errors.
- Impurities: The presence of impurities in either the analyte or the titrant can interfere with the reaction and shift the equivalence point. Careful purification of reagents is necessary to achieve accurate results.
- Experimental Errors: Errors in measurement, such as inaccurate volume readings from the buret or imprecise weighing of the analyte, will directly affect the accuracy of the equivalence point determination.
Types of Titrations and their Equivalence Points
The nature of the equivalence point depends on the type of titration being performed:
1. Strong Acid-Strong Base Titration:
In a strong acid-strong base titration, the equivalence point occurs at pH 7. The reaction is essentially complete, and the resulting solution is neutral. The pH changes dramatically around the equivalence point, making it relatively easy to determine.
2. Weak Acid-Strong Base Titration:
The equivalence point in a weak acid-strong base titration occurs at a pH greater than 7. This is because the conjugate base of the weak acid hydrolyzes water, producing hydroxide ions and thus increasing the pH. The pH change around the equivalence point is less dramatic compared to a strong acid-strong base titration, requiring careful indicator selection.
3. Strong Acid-Weak Base Titration:
Conversely, in a strong acid-weak base titration, the equivalence point occurs at a pH less than 7. This is due to the hydrolysis of the conjugate acid of the weak base. Again, the pH change around the equivalence point is less pronounced than in a strong acid-strong base titration.
4. Weak Acid-Weak Base Titration:
Titrating a weak acid with a weak base is less common, as it's difficult to pinpoint the equivalence point accurately. The pH change near the equivalence point is very gradual, and the choice of indicator is critical.
Beyond Acid-Base Titrations: Equivalence Points in Other Titration Types
The concept of the equivalence point extends beyond acid-base titrations. It applies to other types of titrations as well:
1. Redox Titrations:
In redox titrations, the equivalence point represents the complete oxidation or reduction of the analyte. The potential (voltage) of the solution changes significantly near the equivalence point, and potentiometric methods are frequently used for endpoint detection.
2. Complexometric Titrations:
Complexometric titrations involve the formation of a complex between the analyte and the titrant. The equivalence point is reached when all the analyte has formed a complex with the titrant. Indicators that change color upon complex formation are used to signal the endpoint.
3. Precipitation Titrations:
In precipitation titrations, the equivalence point corresponds to the complete precipitation of the analyte. The sudden decrease in the concentration of the analyte ions signals the equivalence point. However, determining the equivalence point can be challenging due to the slow rate of precipitation in some cases.
Determining the Equivalence Point: Techniques and Methods
Various techniques can be employed to determine the equivalence point:
- Visual Indicators: This is the most common method, relying on the color change of an indicator. The choice of indicator is crucial for accurate results.
- pH Meter: A pH meter allows for continuous monitoring of the pH during the titration. The equivalence point is identified by plotting the pH versus the volume of titrant added, obtaining a titration curve. The equivalence point is the midpoint of the steepest part of the curve.
- Potentiometry: This involves measuring the potential difference (voltage) between an indicator electrode and a reference electrode. The potential changes significantly near the equivalence point in redox and complexometric titrations.
- Conductivity Measurement: In some titrations, the conductivity of the solution changes significantly near the equivalence point, allowing for its determination using a conductivity meter.
Conclusion: The Equivalence Point – A Cornerstone of Quantitative Analysis
The equivalence point is a fundamental concept in titration and a cornerstone of quantitative analysis. Understanding its significance, the factors influencing its determination, and the various methods used to identify it are crucial for conducting accurate and reliable titrations. The careful selection of appropriate techniques, indicators, and a keen understanding of the chemical reaction taking place are all paramount to obtaining precise results. By mastering these principles, analysts can leverage the power of titrations to determine the concentrations of a wide array of substances with high accuracy and precision. The precision obtained via titration makes it a ubiquitous technique employed across various fields including pharmaceutical, environmental, food, and industrial chemistry.
Latest Posts
Latest Posts
-
X 3 3x 2 4x 12 0
Mar 23, 2025
-
How To Calculate Mass Of A Liquid
Mar 23, 2025
-
Is Hbr An Acid Or Base
Mar 23, 2025
-
2 5 6 As An Improper Fraction
Mar 23, 2025
-
10 Gallons Is How Many Quarts
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about What Is The Equivalence Point In A Titration . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
